GOMEKLI (mirdametinib) has been proven effective in adults with NF1-PN
Actor portrayal.
What is GOMEKLI?
GOMEKLI is a prescription medicine used to treat adults and children 2 years of age and older with neurofibromatosis type 1 (NF1) who have plexiform neurofibromas (PN) that cause symptoms and cannot be completely removed by surgery.
It is not known if GOMEKLI is safe and effective in children younger than 2 years of age.
GOMEKLI is a MEK inhibitor, which is targeted therapy—not traditional chemotherapy. It works by helping to block certain signals in the body that cause plexiform neurofibromas to grow.

GOMEKLI has been proven to shrink plexiforms in adults (age 18+) with NF1-PN
GOMEKLI was studied in one of the largest (N=114) clinical trials for neurofibromatosis type 1 with plexiform neurofibromas (NF1-PN).
The single-arm trial enrolled 58 adults (age 18+) and 56 children/adolescents (age 2-17), including some who already had undergone at least 1 PN-related surgery. At the start of the trial, pain and changes in appearance were the most common PN-related symptoms reported by adults and children.
41% of adults
SAW THEIR PLEXIFORM SHRINK by at least 20%*
15 of these adults
SAW THEIR PLEXIFORM SHRINK
by more than 50%†
Plexiform before starting GOMEKLI
20%
SMALLER
50%
SMALLER
*Reduction in plexiform size had to be confirmed on a later magnetic resonance imaging (MRI) scan.
†The 50% reduction in plexiform size was an exploratory analysis, meaning it was not specifically defined in advance of the trial.
Plexiform is short for plexiform neurofibroma, which can also be called a PN.
Meet Austin
Meet Austin
Hear Austin talk about his experience on GOMEKLI and how it has helped him show up, even during the hardest moments.
The video title and GOMEKLI indication statement appear onscreen with the GOMEKLI logo small in the lower right corner.
Text onscreen:
Meet Austin:
GOMEKLI Guide Patient Ambassador
Text onscreen and voice-over:
What is GOMEKLI?
GOMEKLI (mirdametinib) is a prescription medicine used to treat adults and children 2 years of age and older with neurofibromatosis type 1 (NF1) who have plexiform neurofibromas (PN) that cause symptoms and cannot be completely removed by surgery.
It is not known if GOMEKLI is safe and effective in children under 2 years of age.
Image onscreen:
We follow Austin from behind as he walks into a church, past stained-glass windows, and then down a flight of stairs. We then see him seated at a conference table in a smaller room, looking at a bible, and writing notes in his notebook.
[Austin]
Ministry for me is about showing up for people in their hardest moments. I am there as the shepherd, I’m there to remind them and guide them through those hard times. I’ve been there, I’ve done that, so I know what it means to carry something heavy, invisible, and lifelong. I’ve lived with neurofibromatosis type 1 with plexiform neurofibromas, NF1-PN, for almost my entire life. NF1 is a genetic disorder that causes non-cancerous tumors to grow along the nerves throughout the body.
Image onscreen:
Austin is seated in an interview chair, talking to at an interviewer off-camera. The background shows a living space with plants, a bookshelf, coffee table, artwork, rug, French doors, and a window. A name super graphic appears onscreen.
Text onscreen:
Austin
Living with NF1-PN and taking GOMEKLI® (mirdametinib)
Austin is being compensated by SpringWorks Therapeutics, Inc.
[Austin]
I was two and a half years old when my parents noticed a dark discoloration on my back that looked like a bruise. When it didn't go away, they took me to the doctor. Eventually, scans revealed it was a type of tumor called plexiform neurofibroma and I was diagnosed with NF1-PN. That was the beginning of this journey.
Image onscreen:
Austin is seated at a conference table in a small room. He reads a bible and makes notes in his notebook. There is a mug of tea on the table. In the background are stained-glass windows, a lamp, and a couch. Closeup shots of Austin’s hand holding a pen and writing.
[Austin]
NF1 runs in my family. My dad and grandmother both have it. But no one in the family has tumors like me. By the time I was four, I had to have surgery to remove part of it.
Image onscreen:
Austin is seated in an interview chair, talking to at an interviewer off-camera.
[Austin]
Later that year, I needed another surgery to remove more of that tumor because it was pressing back into my spine. We named my tumor Ralph the Runaway Mouse after one of my favorite children books. It helped us talk about it and normalize it. He’s just a part of me, so to speak.
Image onscreen:
Austin walks along some church pews. He is then shown seated, looking up towards the front of the church, then down at the floor. The video cuts back to the interview shot before showing Austin in the church again.
[Austin]
I was already learning that this condition was not going to be easy to live with. I remember being scared about a few things, but my parents were always honest with me about what was going on. Sometimes doctors would try to pull my parents out of the room, but my parents would tell them that “Austin has to live with this, so he needs to be involved in the conversation.” It helped me be somebody that speaks up and advocates for myself.
Image onscreen:
Austin stands in front of a large table in a room in his house. On the table there are a variety of items associated with camping, such as a lantern, first aid kit, backpack, tarp, tent, etc. He checks over the various items. He then consults a map, planning his hiking route. He packs the items in his backpack, puts on his backpack, grabs some other gear, and then exits his house through the front door. The video occasionally cuts back and forth with the interview shot.
[Austin]
Growing up, NF1-PN affected almost everything in my life. I could not always keep up physically with kids my age, so there was a lot of misunderstanding with coaches and teachers. I remember one time, at a basketball game that I got hit hard right in my tumor. And getting yelled at to get right back up when I was in excruciating pain. People don’t always see what you are carrying. When I got a little older, I started talking to doctors myself. I would tell them where it hurt, and what activities would make it worse. My NF1 specialist, treated me like a person, not just another patient. He understood that I know my own body better than anyone and pushed me to find a treatment that worked for me. We tried several pain regulation treatments to try to manage the symptoms that my plexiforms caused. There were a few years in my early twenties when I was not on treatment. My pain was manageable; I just needed a break from all the appointments. But eventually the pain came back. I was having spasms, and the pain was so intense at times, I could hardly move around. I decided to reengage with care and started seeing a new doctor.
Image onscreen:
Austin carries his backpack and gear outside towards the back of his SUV. The rear lift gate opens automatically, and Austin puts his gear in the trunk. As the trunk closes, the GOMEKLI indication statement appears along the bottom of the screen.
[Austin]
That’s when I heard about a clinical trial for a treatment called mirdametinib, what is now GOMEKLI.
Text onscreen:
GOMEKLI (mirdametinib) is a prescription medicine used to treat adults and children 2 years of age and older with neurofibromatosis type 1 (NF1) who have plexiform neurofibromas that cause symptoms and cannot be completely removed by surgery. It is not known if GOMEKLI is safe and effective in children under 2 years of age.
Image onscreen:
Austin is seated in an interview chair, talking to at an interviewer off-camera. The video then cuts to Austin standing in the forest as he consults his map and compass.
[Austin]
My doctor told me the treatment goal of GOMEKLI was to reduce the size of my plexiform tumors by at least 20%.
Image onscreen:
Austin is seated in an interview chair, talking to at an interviewer off-camera. A disclaimer graphic appears along the bottom of the screen. The video then cuts to a shot following Austin as he hikes along a trail in the forest.
Text onscreen:
Please see Important Safety Information throughout and Full Prescribing Information including Patient Information.
[Austin]
When I heard that, I was just astonished. I was in. I was excited about the idea of my tumor shrinking and hoped that it would relieve some of the symptoms I had because of my tumor.
Image onscreen:
Austin is seated in an interview chair, talking to at an interviewer off-camera. A disclaimer graphic appears along the bottom of the screen.
Text onscreen:
The most common side effects of GOMEKLI in adults include: diarrhea, nausea, muscle, joint, and bone pain, vomiting, tiredness. These are not all of the possible side effects of GOMEKLI. Call your doctor for medical advice about side effects.
[Austin]
Starting GOMEKLI was a commitment. 21 days on, 7 days off, with regular MRIs, and side effects such as rashes and digestive issues, but I worked closely with my doctor to manage these. I learned what worked for me such as staying hydrated and using baby lotion on the rash.
Image onscreen:
We see Austin from the front and side as he hikes along a path in the forest. He uses hiking poles to help with stability.
Text onscreen:
Individual results may vary.
[Austin]
And then, after a year on GOMEKLI, we got the results. A 57% reduction in the size of my tumor.
Image onscreen:
Austin is seated in an interview chair, talking to at an interviewer off-camera. The video then cuts to a wide profile shot of Austin hiking through some long grass, with a forest visible in the background.
[Austin]
I was speechless. It was the first time that something has made a noticeable difference in my NF1-PN. Those results really motivated me to stay on GOMEKLI. This is just my experience, and everyone’s experience may be different.
Image onscreen:
We see Austin in a closeup shot as he drinks water from a reusable water bottle. He then walks into his campsite and sets down a bag on a folding chair. His tent is visible in the background, which is set out on some grass surrounded by trees and bushes. There is a small folding table in front of his chair, as well as a metal firepit stocked with firewood. Austin sets up a portable gas stove and pours water into a pot, which he then puts on the stove to boil. He checks on the water and then sits in his chair, waiting for the water to boil. The video then briefly cuts back to the interview shot.
[Austin]
Now, GOMEKLI is just part of my daily routine. I take it once in the morning and once at night. It doesn’t interfere with my work or the way I want to live my life. If there’s something I could say to someone who is newly diagnosed with NF1-PN, find a doctor who listens to you. Speak up, even if you’re not sure that it matters. And you’re not alone. Reach out to others that are living with NF1-PN. Theres a whole community of us out here that understands.
Image onscreen:
Austin stands at a pulpit in a church and addresses his congregation. Behind Austin, we see lots of dark wood paneling, as well as the wall-mounted silver pipes of an organ. Stained glass windows line the sides of the room. Some shots show the backs of heads and shoulders of people as they listen to Austin preach. The video then fades to white.
[Austin]
Ministry is about being real with people. I show up not in spite of my NF1-PN, but because of it. I know pain, I know frustration. On treatment with GOMEKLI, my tumor is smaller. I have the strength and confidence to focus on the things I love.
Image onscreen:
A disclaimer with the title “Important Safety Information” fades onscreen with the GOMEKLI logo small in the lower right corner. The title and GOMEKLI logo stay in place as the text on screen changes as the presentation of Important Safety Information progresses.
Text onscreen and voice-over:
Important Safety Information
Before taking GOMEKLI, tell your healthcare provider about all of your medical conditions, including if you:
- Have eye problems
- Have heart problems
- Are pregnant or plan to become pregnant. GOMEKLI can harm your unborn baby
Females who are able to become pregnant:- Your healthcare provider should check to see if you are pregnant before you begin treatment with GOMEKLI.
- Use effective birth control (contraception) during treatment with GOMEKLI and for 6 weeks after your last dose.
- Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with GOMEKLI.
- Use effective birth control (contraception) during treatment with GOMEKLI and for 3 months after your last dose.
- Talk to your healthcare provider about the best way to feed your baby during this time.
- Are breastfeeding or plan to breastfeed. It is not known if GOMEKLI passes
into your breastmilk.
- Do not breastfeed during treatment with GOMEKLI and for 1 week after your last dose.
- Talk to your healthcare provider about the best way to feed your baby during this time.
How should I take GOMEKLI?
- Take GOMEKLI exactly as your healthcare provider tells you to take it. Your healthcare provider may change your dose, temporarily stop, or permanently stop treatment with GOMEKLI if you develop certain side effects.
- Take GOMEKLI twice a day, about 12 hours apart, for 21 days, followed by 7 days off treatment, to complete a 28-day treatment cycle. Your healthcare provider will decide how many treatment cycles are right for you.
- Take GOMEKLI with or without food.
- GOMEKLI comes in two different dosage forms, GOMEKLI capsules and GOMEKLI tablets for oral suspension. Your healthcare provider will decide the dosage form and dose of GOMEKLI that is right for you.
- If you take GOMEKLI capsules: Swallow each capsule whole with drinking water. If more than 1 capsule is required, swallow 1 capsule at a time. Do not open, break or chew the capsules.
- If you take GOMEKLI tablets for oral suspension, either:
- Swallow each tablet for oral suspension whole with drinking water. If more than 1 tablet is required, swallow 1 tablet at a time.
- Disperse the tablets for oral suspension in drinking water to make a liquid (suspension) before you take or give GOMEKLI. See the “Instructions for Use” that come with your medicine for instructions on how to prepare and take GOMEKLI tablets for oral suspension.
- If you miss a dose of GOMEKLI, skip the missed dose and take your next dose at your regularly scheduled time.
- If you vomit at any time after taking GOMEKLI, do not take an additional dose. Take your next dose at your regularly scheduled time.
What are the possible side effects of GOMEKLI?
GOMEKLI may cause serious side effects, including:
- Eye problems. GOMEKLI may cause eye problems that can lead to blindness.
Your healthcare provider will check your vision before and during treatment
with GOMEKLI. Tell your healthcare provider right away if you get any of the
following signs or symptoms of eye problems:
- Blurred vision
- Loss of vision
- Other changes to your vision
- Heart problems. GOMEKLI may lower the amount of blood pumped by your heart,
which is common in children during treatment with GOMEKLI and can also be
severe. Your healthcare provider will do tests before you start GOMEKLI
treatment, every 3 months during your first year of treatment, and then as
needed to make sure your heart is working properly. Tell your healthcare
provider right away if you get any of the following signs or symptoms of
heart problems:
- Coughing or wheezing
- Shortness of breath
- Swelling of your ankles and feet
- Tiredness
- Increased heart rate
- Skin problems. Skin rashes are common with GOMEKLI in both adults and
children and can also be severe. GOMEKLI can also cause hair loss
(alopecia). Tell your healthcare provider if you develop any of the
following signs or symptoms of skin problems:
- Flat skin rash
- Raised bumps on the skin
- Skin bumps that look like acne
- Skin redness
- Itchy rash
- Peeling skin
- Diarrhea
- Nausea
- Muscle, joint, and bone pain
- Vomiting
- Tiredness
The most common side effects of GOMEKLI in children include:
- Diarrhea
- Muscle, joint, and bone pain
- Stomach (abdominal) pain
- Vomiting
- Headache
- Skin redness, swelling, or pain around the fingernails or toenails
- Nausea
GOMEKLI may cause fertility problems in females, which may affect your ability to have children. Talk to your healthcare provider if you have concerns about fertility.
These are not all of the possible side effects of GOMEKLI. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Please see full Prescribing Information, including Patient Information and Instructions for Use.
Image onscreen:
The GOMEKLI logo appears large in the center of the screen with disclaimer information in a single line along the bottom.
Text onscreen:
©2025 SpringWorks Therapeutics, Inc. All rights reserved. GOMEKLI is a registered trademark of SpringWorks Therapeutics, Inc. C_GOM_US_0332 07/25
Hear Austin talk about his experience on GOMEKLI and how it has helped him show up, even during the hardest moments.
I experienced good results with GOMEKLI. After about 1 year on treatment, my doctor shared that my plexiform had shrunk by 57%.
– Austin, GOMEKLI Patient Ambassador
Individual results may vary.
Ready to share your experience on GOMEKLI?
The GOMEKLI Patient Ambassador Program offers a meaningful opportunity to raise awareness and share your story—whether you’re taking GOMEKLI or caring for someone on treatment.‡
Call or email today to share your story.
In adults who benefited from treatment, plexiforms started to shrink within months of taking GOMEKLI
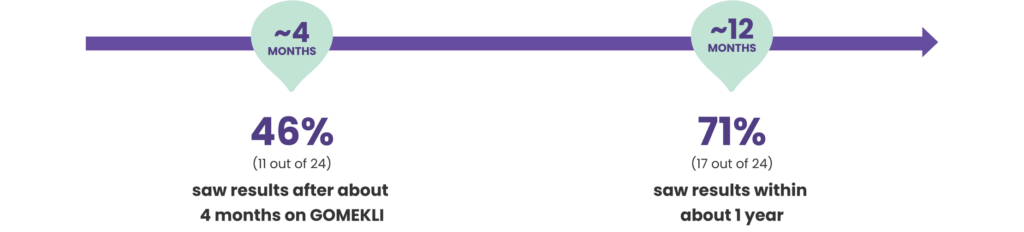
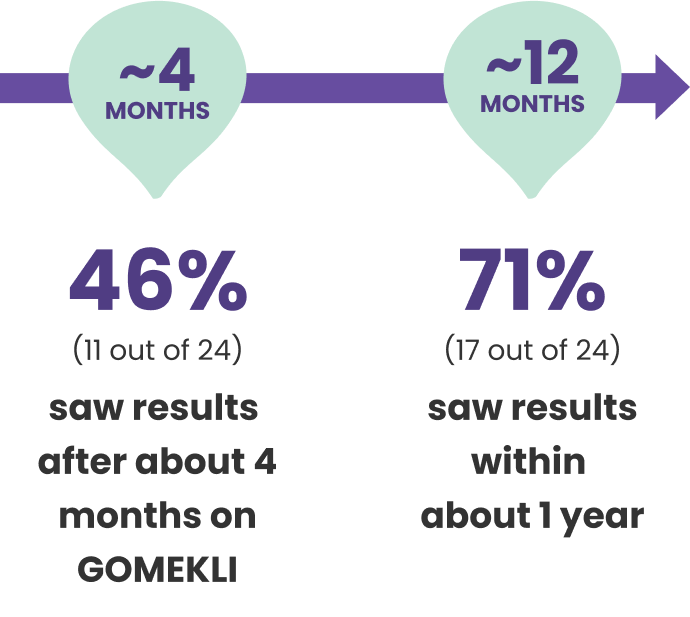
In adults who benefited from treatment, plexiforms began to shrink by at least 20% within 4 to 19 months of starting GOMEKLI. The median (middle) amount of time it took for plexiforms to begin shrinking was 7.8 months.
84% of eligible adults (26 out of 31) chose to keep taking GOMEKLI in an optional long-term follow-up phase of the study.
The GOMEKLI clinical trial was about 2 years long. After that, 84% of eligible adults chose to keep taking GOMEKLI in an optional long-term follow-up phase of the study.
Possible side effects with GOMEKLI (mirdametinib)
GOMEKLI may cause serious side effects, including:
Eye problems
Tell your healthcare provider right away if you get any of the following signs or symptoms of eye problems:
- Blurred vision, loss of vision, or other changes to your vision
Heart problems
Tell your healthcare provider right away if you get any of the following signs or symptoms of heart problems:
- Coughing or wheezing, shortness of breath, swelling of your ankles and feet, tiredness, increased heart rate
Skin problems
Tell your healthcare provider if you develop any of the following signs or symptoms of skin problems:
- Flat skin rash, raised bumps on the skin, skin bumps that look like acne, skin redness, itchy rash, peeling skin
In adults, the most common ones were:
- Diarrhea
- Nausea
- Muscle, joint, and bone pain
- Vomiting
- Tiredness
The most common severe abnormal blood test result in adults involved an increased enzyme called creatine phosphokinase (CPK).
These are not all the possible side effects of GOMEKLI. Call your doctor for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088.
Most adults taking GOMEKLI did not have to stop treatment due to a side effect
had to stop treatment due to a side effect
Your healthcare provider may interrupt, reduce, or permanently stop GOMEKLI treatment if you experience certain side effects.
Managing side effects
Managing side effects
Learn how to work with your care team to manage the possible side effects of GOMEKLI.
Petal shape from GOMEKLI logo appears onscreen, spinning as if the video is loading. Petal shape comes to a stop as full GOMEKLI logo fades in.
Image onscreen:
GOMEKLI logo shrinks and moves to top center of the frame. Indication statement appears.
Text onscreen and voice-over:
GOMEKLI (mirdametinib) is a prescription medicine used to treat adults and children 2 years of age and older with neurofibromatosis type 1 (NF1) who have plexiform neurofibromas (PN) that cause symptoms and cannot be completely removed by surgery.
It is not known if GOMEKLI is safe and effective in children under 2 years of age.
Please see Important Safety Information later in this video and Patient Information available on GOMEKLI.com.
Image onscreen:
GOMEKLI logo fades and a flag with the logo in the top right corner of the frame appears, where it remains for the entirety of the video. Title of video fades in.
Text onscreen:
Managing possible side effects of GOMEKLI
Voice-over:
In this video, we’ll talk about how to work with your care team to manage common side effects and offer tips that may help throughout treatment.
Image onscreen:
Word bubbles pop up around a woman as the different side effects are introduced. The woman shrinks and moves to the bottom left of the screen as the child comes into view. The word bubble bubbles pop up around him as pediatric symptoms are introduced. The shot pans to the woman sitting across from her doctor.
Voice-over:
The most common side effects with GOMEKLI are diarrhea, nausea, muscle, joint, and bone pain, and vomiting. Some adults may also experience tiredness, while some children may experience headache, stomach pain, and skin redness, swelling, or pain around the fingernails and toenails.
GOMEKLI can cause other side effects, including some serious side effects which we will go over in this video.
Most of the side effects associated with GOMEKLI have been shown to start early in treatment, but it’s good to know that there are things your care team can do to help address them.
Image onscreen:
The camera zooms in to the woman’s arm with a rash. Different rash types appear in word bubbles around her as they are introduced.
Text onscreen:
One of the most common side effects with GOMEKLI is rash.
Voice-over:
In the single-arm clinical study of GOMEKLI, one of the most common side effects seen among both adults and children was rash, which can be severe. This could be a flat skin rash, raised bumps on the skin, skin bumps that look like acne, skin redness, itchy rash, or peeling skin.
Image onscreen:
The woman is shown with her doctor as she examines her rash. The screen splits. On the left the woman in a bathtub. On the right, the woman is applying lotion to her arm.
Text onscreen:
This information is not intended to replace the advice of your care team or other healthcare provider.
Voice-over:
There are things you can do to help address skin-related side effects. These include taking daily baths and using mild cleansers and skin moisturizers at least twice a day to prevent dryness. You may also consider avoiding products that could dry out or irritate your skin.
Image onscreen:
A doctor types up a prescription for her patient.
Text onscreen:
Tell your care team if you develop any signs or symptoms of skin problems.
Voice-over:
Your care team may also provide medication to help with the rash or refer you to a dermatologist for additional care.
Image onscreen:
A woman holds her stomach in discomfort. Different types of gastrointestinal appear in word bubbles around her as they are introduced.
Text onscreen:
Diarrhea, nausea, vomiting, and abdominal pain are also common side effects.
Voice-over:
Gastrointestinal side effects, such as diarrhea, nausea, vomiting, and abdominal pain, are also common in people who take GOMEKLI.
Image onscreen:
Food icons of french fries, hot peppers, and potato chips appear on screen in large white circles and lines going through them. They disappear and a glass appears onscreen. The shot pans to a pharmacy where the woman is looking at different medications.
Text onscreen:
This information is not intended to replace the advice of your care team or other healthcare provider.
Voice-over:
There are certain tips you can keep in mind to address stomach-related side effects, such as avoiding fried, fatty, or spicy foods, and increasing your fluid intake. If you continue to experience issues, consult your care team for further treatment options.
Image onscreen:
The woman is shown speaking with her doctor. The doctor nods and takes notes.
Text onscreen:
Take GOMEKLI as directed by your healthcare provider.
Voice-over:
Depending on what you’re experiencing, your care team may interrupt, reduce, or permanently stop your GOMEKLI treatment. It is important that you take GOMEKLI as directed by your healthcare provider.
Image onscreen:
The woman nods her head as she continues speaking with her doctor. The serious side effects pop up in word bubbles around her. The doctor then begins to examine her eyes.
Text onscreen:
GOMEKLI can cause eye and heart problems that can be serious.
Voice-over:
Because GOMEKLI can cause serious side effects, including eye problems and heart problems, your care team will regularly assess your eyes and heart before and throughout treatment. Before taking GOMEKLI, make sure to tell your healthcare provider if you have any eye or heart problems.
Image onscreen:
The doctor and woman discuss her lab results.
Text onscreen:
Your care team will conduct blood tests.
Voice-over:
Your care team will also want to run routine lab work, including blood tests. These assessments are important because they help your care team see if anything is going on that needs to be addressed.
Image onscreen:
The woman checks her phone. The camera zooms in on her screen, where it shows her scrolling through her contact list.
Text onscreen:
Regular check-ins with your care team are extremely important.
Voice-over:
That’s why it’s vital to stay on top of appointments.
Image onscreen:
The woman clicks on the contact for her care team. The screen splits: On the left the woman has the phone to her ear and on the right a doctor also has a phone to her ear.
Text onscreen:
Tell your care team immediately if you notice anything out of the ordinary.
Voice-over:
But remember, if you’re experiencing anything out of the ordinary, you shouldn’t wait until your next appointment to let your care team know. The key to staying on top of side effects is being proactive, open, and honest with your care team. They won’t know how to help you if you don’t speak up!
Image onscreen:
The GOMEKLI treatment journal appears. The pages turn so viewers can see what it looks like and how it works. We fade to the woman sitting at a desk reading through the treatment journal.
Text onscreen:
Use the treatment journal to track your appointments, how you’re feeling, and more.
Voice-over:
The GOMEKLI treatment journal can make things easier. It includes weekly templates so you can record how you’re feeling, jot down general notes, and save any questions you have for your care team. Once you start GOMEKLI and are enrolled in SpringWorks CareConnections, the journal will be sent to you as part of your GOMEKLI starter kit. You can also find it on GOMEKLI.com.
Image onscreen:
The treatment journal goes away and is replaced with a laptop. On the laptop screen is GOMEKLI.com resource page, which is being scrolled through.
Text onscreen:
Find more helpful resources on GOMEKLI.com.
Voice-over:
GOMEKLI.com also has other helpful resources in case you have questions about treatment.
Image onscreen:
The woman continues to explore GOMEKLI.com and makes her way to the SpringWorks CareConnections® page.
Text onscreen:
The SpringWorks CareConnections® Patient Support Program is not intended to take the place of your healthcare provider, and our team of Nurse Advocates cannot provide medical or clinical advice.
Voice-over:
Additionally, once you’re enrolled in SpringWorks CareConnections, our team of Nurse Advocates will be available to provide support and help you stay on track with treatment.
Image onscreen:
QUICK CUTS: Woman takes notes in her journal. She calls her doctor. Doctor is shown speaking on the phone.
Voice-over:
Managing side effects is a team effort involving both you and your care team. Keeping an open line of communication is key to ensuring you have the best experience possible with GOMEKLI. If you have any questions or concerns, please reach out to your healthcare provider. Keep watching for additional important information on side effects with GOMEKLI.
Text onscreen and voice-over:
Important Safety Information
Before taking GOMEKLI, tell your healthcare provider about all of your medical conditions, including if you:
- Have eye problems
- Have heart problems
-
Are pregnant or plan to become pregnant. GOMEKLI can harm your unborn baby
- Your healthcare provider should check to see if you are pregnant before you begin treatment with GOMEKLI.
- Use effective birth control (contraception) during treatment with GOMEKLI and for 6 weeks after your last dose.
- Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with GOMEKLI. Males with female partners who are able to become pregnant:
- Use effective birth control (contraception) during treatment with GOMEKLI and for 3 months after your last dose.
- Tell your healthcare provider right away if your female partner becomes pregnant or thinks she may be pregnant during treatment with GOMEKLI.
Females who are able to become pregnant: -
Are breastfeeding or plan to breastfeed. It is not known if GOMEKLI passes
into your breastmilk.
- Do not breastfeed during treatment with GOMEKLI and for 1 week after your last dose.
- Talk to your healthcare provider about the best way to feed your baby during this time.
Tell your healthcare provider about all the medicines you take, including
prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I take GOMEKLI?
- Take GOMEKLI exactly as your healthcare provider tells you to take it. Your healthcare provider may change your dose, temporarily stop, or permanently stop treatment with GOMEKLI if you develop certain side effects.
- Take GOMEKLI twice a day, about 12 hours apart, for 21 days, followed by 7 days off treatment, to complete a 28-day treatment cycle. Your healthcare provider will decide how many treatment cycles are right for you.
- Take GOMEKLI with or without food.
- GOMEKLI comes in two different dosage forms, GOMEKLI capsules and GOMEKLI tablets for oral suspension. Your healthcare provider will decide the dosage form and dose of GOMEKLI that is right for you.
- If you take GOMEKLI capsules: Swallow each capsule whole with drinking water. If more than 1 capsule is required, swallow 1 capsule at a time. Do not open, break or chew the capsules.
-
If you take GOMEKLI tablets for oral suspension, either:
- Swallow each tablet for oral suspension whole with drinking water. If more than 1 tablet is required, swallow 1 tablet at a time. OR
- Disperse the tablets for oral suspension in drinking water to make a liquid (suspension) before you take or give GOMEKLI.
See the “Instructions for Use” that come with your medicine for instructions
on how to prepare and take GOMEKLI tablets for oral suspension.
- If you miss a dose of GOMEKLI, skip the missed dose and take your next dose at your regularly scheduled time.
- If you vomit at any time after taking GOMEKLI, do not take an additional dose. Take your next dose at your regularly scheduled time.
What are the possible side effects of GOMEKLI?
GOMEKLI may cause serious side effects, including:
-
Eye problems. GOMEKLI may cause eye problems that can lead to blindness.
Your healthcare provider will check your vision before and during treatment
with GOMEKLI. Tell your healthcare provider right away if you get any of the
following signs or symptoms of eye problems:
- Blurred vision
- Loss of vision
- Other changes to your vision
-
Heart problems. GOMEKLI may lower the amount of blood pumped by your heart,
which is common in children during treatment with GOMEKLI and can also be
severe. Your healthcare provider will do tests before you start GOMEKLI
treatment, every 3 months during your first year of treatment, and then as
needed to make sure your heart is working properly. Tell your healthcare
provider right away if you get any of the following signs or symptoms of
heart problems:
- Coughing or wheezing
- Shortness of breath
- Swelling of your ankles and feet
- Tiredness
- Increased heart rate
-
Skin problems. Skin rashes are common with GOMEKLI in both adults and
children and can also be severe. GOMEKLI can also cause hair loss
(alopecia). Tell your healthcare provider if you develop any of the
following signs or symptoms of skin problems:
- Flat skin rash
- Raised bumps on the skin
- Skin bumps that look like acne
- Skin redness
- Itchy rash
- Peeling skin
The most common side effects of GOMEKLI in adults include:
- Diarrhea
- Nausea
- Muscle, joint, and bone pain
- Vomiting
- Tiredness
The most common severe abnormal blood tests in adults include an increased enzyme called creatine phosphokinase (CPK).
The most common side effects of GOMEKLI in children include:
- Diarrhea
- Muscle, joint, and bone pain
- Stomach (abdominal) pain
- Vomiting
- Headache
- Skin redness, swelling, or pain around the fingernails or toenails
- Nausea
The most common severe abnormal blood tests in children include decreased white blood cell (neutrophil) counts and increased CPK.
GOMEKLI may cause fertility problems in females, which may affect your ability to have children. Talk to your healthcare provider if you have concerns about fertility.
These are not all of the possible side effects of GOMEKLI. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Please see full Prescribing Information, including Patient Information and Instructions for Use, available on GOMEKLI.com.
Text onscreen:
Thank you!
©2025 SpringWorks Therapeutics, Inc. All rights reserved. GOMEKLI and SpringWorks Therapeutics are registered trademarks of SpringWorks Therapeutics, Inc. C_GOM_US_0432 8/25
Voice-over:
Thank you for watching.
Learn how to work with your care team to manage the possible side effects of GOMEKLI.
During the first couple months of treatment, I experienced a rash and diarrhea, but I was able to manage my side effects by working with my doctor, applying baby lotion to the rash, and staying hydrated to help with my diarrhea.
– Austin, GOMEKLI Patient Ambassador
Find the care you need
Is GOMEKLI right for me?
This guide can help you and your healthcare provider understand if GOMEKLI could be right for you.
Dosing that fits your needs
Available in capsules or tablets for oral suspension that give you the option to take GOMEKLI as a liquid.
What is GOMEKLI?
It is not known if GOMEKLI is safe and effective in children under 2 years of age.
Important Safety Information
Before taking GOMEKLI, tell your healthcare provider about all of your medical conditions, including if you:
- Have eye problems
- Have heart problems
- Are pregnant or plan to become pregnant. GOMEKLI can harm your unborn baby
Females who are able to become pregnant:
- Your healthcare provider should check to see if you are pregnant before you begin treatment with GOMEKLI.
- Use effective birth control (contraception) during treatment with GOMEKLI and for 6 weeks after your last dose.
- Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with GOMEKLI.
Males with female partners who are able to become pregnant:
- Use effective birth control (contraception) during treatment with GOMEKLI and for 3 months after your last dose.
- Tell your healthcare provider right away if your female partner becomes pregnant or thinks she may be pregnant during treatment with GOMEKLI.
- Are breastfeeding or plan to breastfeed. It is not known if GOMEKLI passes into your breastmilk.
- Do not breastfeed during treatment with GOMEKLI and for 1 week after your last dose.
- Talk to your healthcare provider about the best way to feed your baby during this time.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I take GOMEKLI?
- Take GOMEKLI exactly as your healthcare provider tells you to take it. Your healthcare provider may change your dose, temporarily stop, or permanently stop treatment with GOMEKLI if you develop certain side effects.
- Take GOMEKLI twice a day, about 12 hours apart, for 21 days, followed by 7 days off treatment, to complete a 28-day treatment cycle. Your healthcare provider will decide how many treatment cycles are right for you.
- Take GOMEKLI with or without food.
- GOMEKLI comes in two different dosage forms, GOMEKLI capsules and GOMEKLI tablets for oral suspension. Your healthcare provider will decide the dosage form and dose of GOMEKLI that is right for you.
- If you take GOMEKLI capsules: Swallow each capsule whole with drinking water. If more than 1 capsule is required, swallow 1 capsule at a time. Do not open, break or chew the capsules.
- If you take GOMEKLI tablets for oral suspension, either:
- Swallow each tablet for oral suspension whole with drinking water. If more than 1 tablet is required, swallow 1 tablet at a time.
- Disperse the tablets for oral suspension in drinking water to make a liquid (suspension) before you take or give GOMEKLI.
OR
See the “Instructions for Use” that come with your medicine for instructions on how to prepare and take GOMEKLI tablets for oral suspension.
- If you miss a dose of GOMEKLI, skip the missed dose and take your next dose at your regularly scheduled time.
- If you vomit at any time after taking GOMEKLI, do not take an additional dose. Take your next dose at your regularly scheduled time.
What are the possible side effects of GOMEKLI?
- Eye problems. GOMEKLI may cause eye problems that can lead to blindness. Your healthcare provider will check your vision before and during treatment with GOMEKLI. Tell your healthcare provider right away if you get any of the following signs or symptoms of eye problems:
- Blurred vision
- Loss of vision
- Other changes to your vision
- Heart problems. GOMEKLI may lower the amount of blood pumped by your heart, which is common in children during treatment with GOMEKLI and can also be severe. Your healthcare provider will do tests before you start GOMEKLI treatment, every 3 months during your first year of treatment, and then as needed to make sure your heart is working properly. Tell your healthcare provider right away if you get any of the following signs or symptoms of heart problems:
- Coughing or wheezing
- Shortness of breath
- Swelling of your ankles and feet
- Tiredness
- Increased heart rate
- Skin problems. Skin rashes are common with GOMEKLI in both adults and children and can also be severe. GOMEKLI can also cause hair loss (alopecia). Tell your healthcare provider if you develop any of the following signs or symptoms of skin problems:
- Flat skin rash
- Raised bumps on the skin
- Skin bumps that look like acne
- Skin redness
- Itchy rash
- Peeling skin
The most common side effects of GOMEKLI in adults include:
- Diarrhea
- Nausea
- Muscle, joint, and bone pain
- Vomiting
- Tiredness
The most common side effects of GOMEKLI in children include:
- Diarrhea
- Muscle, joint, and bone pain
- Stomach (abdominal) pain
- Vomiting
- Headache
- Skin redness, swelling, or pain around the fingernails or toenails
- Nausea
What is GOMEKLI?
It is not known if GOMEKLI is safe and effective in children under 2 years of age.
What does confirmed overall response mean?
In clinical trials, endpoints are used to measure if a treatment is working. The primary endpoint of this trial was called confirmed overall response rate (ORR).
In the GOMEKLI (mirdametinib) trial, an overall response meant that a person treated with GOMEKLI had a plexiform shrink by 20% or more. The “confirmed” part means that another scan done later also showed that the plexiform stayed smaller.
The confirmed ORR for GOMEKLI tells us how many trial participants saw their plexiform shrink by 20% or more in 2 back-to-back scans. “41% confirmed ORR” means that 24 of the 58 adults who received GOMEKLI had a plexiform shrink by at least 20% and stay smaller on the next scan.
Why 20%?
The 20% change in plexiform volume is the standard benchmark used in NF1-PN clinical trials.
You are now leaving GOMEKLI.com
You are now leaving GOMEKLI.com, a website provided by SpringWorks Therapeutics. This link will take you to a different site to which this Privacy Policy and Terms of Use do not apply.
This site is intended for US Healthcare Professionals
Tap "Continue" if you are a US Healthcare Professional.