Hear about GOMEKLI from different perspectives
Actor portrayal.
Getting patients started
Drs. Nghiemphu and Weintraub discuss how to work with patients to set up a positive treatment experience with GOMEKLI.
Text onscreen:
[Title builds on purple background with GOMEKLI logo in top right corner.]
Text onscreen:
Chapter 1: Setting efficacy expectations
Voice-over:
Dr. Nghiemphu: Hello. I’m Dr. Phioanh Leia Nghiemphu, a neuro-oncologist specializing in neurofibromatosis and brain tumors in adults.
Text onscreen:
Phioanh Leia Nghiemphu, MD
Dr. Weintraub: And I’m Dr. Lauren Weintraub, a hematologist and oncologist specializing in pediatric cancers and blood disorders. In this video, we’ll talk about how to support a positive treatment experience for patients taking GOMEKLI.
Text onscreen:
Lauren Weintraub, MD
GOMEKLI is the first FDA-approved treatment for both adults and children 2 years of age and older with neurofibromatosis type 1 who have symptomatic plexiform neurofibromas that are not amenable to complete resection. Important Safety Information for GOMEKLI will be presented later in this video.
Text onscreen:
GOMEKLI is the first FDA-approved treatment for both adults and children 2 years of age and older with neurofibromatosis type 1 (NF1) who have symptomatic plexiform neurofibromas (PN) that are not amenable to complete resection.
Voice-over:
Dr. Nghiemphu: Several factors can drive the decision to use a systemic therapy. I consider how the plexiform neurofibromas are impacting my patient: Are they in pain? Is the plexiform neurofibroma causing changes in appearance? Has the patient’s mobility changed?
The patient and I have a conversation about their options, including surgery. For some patients, the plexiform neurofibromas are so intertwined with the nerves that complete resection is not possible. Other patients don’t want to undergo surgery. Either way, we discuss how an oral systemic treatment may be appropriate.
Voice-over: When discussing GOMEKLI as an option for patients, I like to go over some of the results from the phase 2b, single-arm ReNeu trial. The data can be complex, so I focus on explaining it in a way that’s clear and relatable. I mention that both adults and children with NF1-PN were included, that they received GOMEKLI for about 2 years, and that they had the option to remain on GOMEKLI for long-term follow-up.
Text onscreen:
114 patients
58 adult patients (18 to 69 years)
56 pediatric patients (2 to 17 years)
Voice-over:
Dr. Weintraub: The primary endpoint was confirmed overall response rate, and I explain that a confirmed overall response meant a person treated with GOMEKLI had 2 or more consecutive MRI scans showing that their plexiform shrank by 20% or more.
Text onscreen:
Primary endpoint
Confirmed overall response (ORR), defined as the proportion of patients with complete response (disappearance of the target PN) or partial response (≥20% reduction) on magnetic resonance imaging (MRI) of the target PN volume from baseline to Cycle 24 (treatment phase) as assessed by blinded independent central review (BICR) on ≥2 consecutive scans within 2 to 6 months.
Voice-over:
Dr. Weintraub: I also note that secondary endpoints included duration of response and change in patient-reported outcomes of tumor pain severity, pain interference, and health-related quality of life.
In the ReNeu clinical trial, 41% of adult patients—or 24 out of 58—saw their plexiform neurofibroma shrink by at least 20%. Of these 24 patients, 62% achieved a deep response, meaning their target plexiform neurofibroma decreased by more than half the size it was at baseline.
Text onscreen:
Graphic of confirmed overall response rates in adults.
Voice-over:
Dr. Weintraub: In the pediatric cohort, 52% of patients—or 29 out of 56—achieved a confirmed overall response. Of these 29 patients, more than half—52%—achieved a deep response.
Text onscreen:
Graphic of confirmed overall response rates in children.
Voice-over:
Dr. Weintraub: I think it’s important to set realistic expectations about how GOMEKLI may help, so I tailor the conversation to the individual characteristics and needs of the patient. I also underscore that not everyone will have the same results.
Dr. Nghiemphu: Yeah, it’s so important to remind patients that clinical trial data in the aggregate is one thing and their personal experience on treatment is another. If patients are expecting to see their plexiform neurofibroma get smaller overnight or in a week, they could get discouraged when that doesn’t happen and want to stop taking their medication. That’s definitely something I want to get ahead of before they start treatment. I highlight that while some patients in the ReNeu trial started to see their plexiform neurofibromas shrink within about 4 months of starting GOMEKLI, for others it took longer. For some it took about 8 months or longer to see a 20% or greater reduction on back-to-back MRI scans. But it’s important that patients stay on track and keep taking their medication as prescribed so that they can get the most out of their treatment.
Dr. Weintraub: That’s a great point. Speaking of staying on track, one question that frequently comes up in my conversations with caregivers is how long their child will have to stay on treatment. I tell them that while every patient is unique, I’ve had a number of patients who participated in the ReNeu trial and have remained on GOMEKLI treatment ever since.
Text onscreen:
GOMEKLI treatment should be continued until disease progression or unacceptable toxicity.
Text onscreen:
Chapter 2: Reviewing adverse reactions
Text onscreen:
Dr. Phioanh Leia Nghiemphu and Dr. Lauren Weintraub sitting in chairs
Voice-over:
Dr. Nghiemphu: Explaining side effects is a critical part of the initial treatment conversation. I don’t want to overwhelm patients, but I certainly want them to understand what changes, aside from changes in the size of their plexiform neurofibromas, they may experience.
In ReNeu, the most commonly reported adverse reactions in both adults and children were rash, diarrhea, nausea, musculoskeletal pain, and vomiting. Fatigue was also reported in adults.
Text onscreen:
Chart with adult adverse reaction data
Voice-over:
Dr. Nghiemphu: Abdominal pain, headache, paronychia, and left ventricular dysfunction were also reported in children. The most common severe lab abnormalities were increased creatine phosphokinase, or CPK, in both adults and children and decreased neutrophil count in children. Serious side effects associated with GOMEKLI include eye problems, heart problems, skin problems, and embryo-fetal toxicity.
Text onscreen:
Chart with pediatric adverse reaction data
Voice-over:
Dr. Weintraub: I feel it’s important to set expectations with patients and caregivers regarding skin-related side effects and counsel them on management strategies. It’s important to address any concerns they may have before starting treatment. Trust me, no teenager wants to be dealing with skin issues on top of living with plexiform neurofibromas.
Dr. Nghiemphu: I definitely have similar conversations with my adult patients about the potential skin-related and gastrointestinal side effects they may experience while on GOMEKLI.
Text onscreen:
Of the patients who had dermatologic adverse reactions in ReNeu, the majority (80%) experienced first onset during Cycle 1 of treatment.
Voice-over:
Dr. Nghiemphu: I make sure they understand that many of these side effects were seen early in the course of treatment and that there are ways to help manage them, including over-the-counter remedies, medications, and even small lifestyle changes.
Text onscreen:
Of the patients who had GI adverse reactions in ReNeu, the majority experienced first onset early (Cycles 1-3).
Text onscreen:
Chart detailing the proportion of patients experiencing GI reactions in the first 2 cycles of GOMEKLI treatment.
Voice-over:
Dr. Nghiemphu: I also discuss how important it is that they are proactive and let me know right away if something is going on. The earlier we can treat the side effect, the better chance we have at resolving it quickly. In addition to managing the side effects, it’s important for patients and caregivers to know that interrupting or reducing their GOMEKLI dose is also an option. And if side effects continue after those adjustments, we have the option to permanently stop GOMEKLI.
Dr. Weintraub: Patients and caregivers should also know that certain assessments, like blood and urine tests, are required before and throughout GOMEKLI treatment. Additionally, because GOMEKLI may cause serious issues with the eyes and heart, eye exams and echocardiograms are also required before and throughout treatment.
Text onscreen:
Icons and check marks next to assessments: blood tests, urinalysis, eye exams, and echocardiograms
Voice-over:
Dr. Weintraub: Generally, these assessments are done more frequently at the start of treatment and become less frequent over time, as things remain stable. I think it’s important for patients and their caregivers to know that these routine assessments help me and their care team monitor how they are doing on treatment. The GOMEKLI Dosing and Adverse Reaction Management Guide outlines some steps we can take as care providers to help manage adverse reactions that occur during GOMEKLI treatment.
Text onscreen:
Animated Dosing and Adverse Reaction Management Guide and QR code.
Text onscreen:
Chapter 3: Dosing, administration, and support
Text onscreen:
Dr. Phioanh Leia Nghiemphu and Dr. Lauren Weintraub sitting in chairs
Voice-over:
Dr. Nghiemphu: How a medication is administered is usually one of the first questions patients have when we’re discussing treatment. GOMEKLI is a twice-daily oral medication that can be taken with or without food. It’s available in 2 capsule sizes and as a dispersible tablet for those who have difficulty swallowing.
Dr. Weintraub: Having the dispersible option is terrific for my patients who can’t or don’t want to take pills. I have a preteen patient who doesn’t like pills and the liquid formulation is just so much easier for him. I also know many caregivers prefer the dispersible tablet because it helps with administering the medication to younger patients.
Dr. Nghiemphu: And of course, some patients have plexiform neurofibromas in the head and neck area that can impact their ability to swallow, so having the option of a dispersible tablet is beneficial to them as well. The dosing schedule for GOMEKLI is 3 weeks on, 1 week off.
Text onscreen:
A chart showing the 4-week dosing cycle
Dr. Nghiemphu: For some patients, having that 1 week off is a nice break because they don’t have to worry about taking treatment. The GOMEKLI Digital Companion is a free and secure resource, available on the Medisafe app, that allows patients to set customized medication reminders, so they never miss a dose.
Dr. Weintraub: We know the coordination of starting a treatment can be a lot for patients and caregivers. SpringWorks CareConnections provides personalized support services to help patients get started and stay on track with GOMEKLI. This includes coverage and access support, financial assistance, personalized education, and emotional support.
Text onscreen:
SpringWorks CareConnections logo and a QR code
Dr. Weintraub: There’s also a full library of resources on GOMEKLI.com that can help set patients up for a positive treatment experience.
Text onscreen:
Additional videos and resources to support your practice and your patients are on GOMEKLI.com/hcp.
Text onscreen:
QR code
Voice-over:
Dr. Nghiemphu: I think we can all agree that when starting a new treatment, it’s important to provide patients with as much information as possible to help set them up for success.
Thank you for listening to our discussion of how we support patients when starting their treatment journey with GOMEKLI.
Voice-over:
Indication
GOMEKLI (mirdametinib) is indicated for the treatment of adult and pediatric patients 2 years of age and older with neurofibromatosis type 1 (NF1) who have symptomatic plexiform neurofibromas (PN) not amenable to complete resection.
Important Safety Information
Warning and Precautions
Ocular Toxicity: GOMEKLI can cause ocular toxicity including retinal vein occlusion (RVO), retinal pigment epithelium detachment (RPED), and blurred vision. In the adult pooled safety population, ocular toxicity occurred in 28% of patients treated with GOMEKLI: 21% were Grade 1, 5% were Grade 2 and 1.3% were Grade 3. RVO occurred in 2.7%, RPED occurred in 1.3%, and blurred vision occurred in 9% of adult patients. In the pediatric pooled safety population, ocular toxicity occurred in 19% of patients: 17% were Grade 1 and 1.7% were Grade 2. Conduct comprehensive ophthalmic assessments prior to initiating GOMEKLI, at regular intervals during treatment, and to evaluate any new or worsening visual changes such as blurred vision. Continue, withhold, reduce the dose, or permanently discontinue GOMEKLI as clinically indicated.
Left Ventricular Dysfunction: GOMEKLI can cause left ventricular dysfunction. GOMEKLI has not been studied in patients with a history of clinically significant cardiac disease or LVEF <55% prior to initiation of treatment. In the ReNeu study, decreased LVEF of 10 to <20% occurred in 16% of adult patients treated with GOMEKLI. Five patients (9%) required dose interruption, one patient (1.7%) required a dose reduction, and one patient required permanent discontinuation of GOMEKLI. The median time to first onset of decreased LVEF in adult patients was 70 days. Decreased LVEF of 10 to <20% occurred in 25%, and decreased LVEF of ≥20% occurred in 1.8% of pediatric patients treated with GOMEKLI. One patient (1.8%) required dose interruption of GOMEKLI. The median time to first onset of decreased LVEF in pediatric patients was 132 days. All patients with decreased LVEF were identified during routine echocardiography, and decreased LVEF resolved in 75% of patients. Before initiating GOMEKLI, assess ejection fraction (EF) by echocardiogram. Monitor EF every 3 months during the first year and then as clinically indicated. Withhold, reduce the dose, or permanently discontinue GOMEKLI based on severity of adverse reaction.
Dermatologic Adverse Reactions: GOMEKLI can cause dermatologic adverse reactions including rash. The most frequent rashes included dermatitis acneiform, rash, eczema, maculo-papular rash and pustular rash. In the pooled adult safety population, rash occurred in 92% of patients treated with GOMEKLI (37% were Grade 2 and 8% were Grade 3) and resulted in permanent discontinuation in 11% of patients. In the pooled pediatric safety population, rash occurred in 72% of patients treated with GOMEKLI (22% were Grade 2 and 3.4% were Grade 3) and resulted in permanent discontinuation in 3.4% of patients. Initiate supportive care at first signs of dermatologic adverse reactions. Withhold, reduce the dose, or permanently discontinue GOMEKLI based on severity of adverse reaction.
Embryo-Fetal Toxicity: GOMEKLI can cause fetal harm when administered to a pregnant woman. Verify the pregnancy status of females of reproductive potential prior to the initiation of GOMEKLI. Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Also advise patients to use effective contraception during treatment with GOMEKLI and for 6 weeks after the last dose (females) or 3 months after the last dose (males).
Adverse Reactions
The most common adverse reactions (>25%) in adult patients were rash (90%), diarrhea (59%), nausea (52%), musculoskeletal pain (41%), vomiting (38%), and fatigue (29%). Serious adverse reactions occurred in 17% of adult patients who received GOMEKLI. The most common Grade 3 or 4 laboratory abnormality (>2%) was increased creatine phosphokinase.
The most common adverse reactions (>25%) in pediatric patients were rash (73%), diarrhea (55%), musculoskeletal pain (41%), abdominal pain (39%), vomiting (39%), headache (34%), paronychia (32%), left ventricular dysfunction (27%), and nausea (27%). Serious adverse reactions occurred in 14% of pediatric patients who received GOMEKLI. The most common Grade 3 or 4 laboratory abnormalities (>2%) were decreased neutrophil count and increased creatine phosphokinase.
Use in Specific Populations
Verify the pregnancy status of patients of reproductive potential prior to initiating GOMEKLI. Due to the potential for adverse reactions in a breastfed child, advise patients not to breastfeed during treatment with GOMEKLI and for 1 week after the last dose.
To report SUSPECTED ADVERSE REACTIONS, contact SpringWorks Therapeutics Inc. at 1-888-400-7989 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see full Prescribing Information, including Patient Information and Instructions for Use.
Text onscreen:
GOMEKLI and SpringWorks logos
Text onscreen:
@2025 SpringWorks Therapeutics, Inc. All rights reserved. GOMEKLI and SpringWorks CareConnections are registered trademarks of SpringWorks Therapeutics, Inc. C_GOM_US_0413 8/25
[Title builds on purple background with GOMEKLI logo in top right corner.]
Text onscreen:
Chapter 1: Setting efficacy expectations
Voice-over:
Dr. Nghiemphu: Hello. I’m Dr. Phioanh Leia Nghiemphu, a neuro-oncologist specializing in neurofibromatosis and brain tumors in adults.
Text onscreen:
Phioanh Leia Nghiemphu, MD
Dr. Weintraub: And I’m Dr. Lauren Weintraub, a hematologist and oncologist specializing in pediatric cancers and blood disorders. In this video, we’ll talk about how to support a positive treatment experience for patients taking GOMEKLI.
Text onscreen:
Lauren Weintraub, MD
GOMEKLI is the first FDA-approved treatment for both adults and children 2 years of age and older with neurofibromatosis type 1 who have symptomatic plexiform neurofibromas that are not amenable to complete resection. Important Safety Information for GOMEKLI will be presented later in this video.
Text onscreen:
GOMEKLI is the first FDA-approved treatment for both adults and children 2 years of age and older with neurofibromatosis type 1 (NF1) who have symptomatic plexiform neurofibromas (PN) that are not amenable to complete resection.
Voice-over:
Dr. Nghiemphu: Several factors can drive the decision to use a systemic therapy. I consider how the plexiform neurofibromas are impacting my patient: Are they in pain? Is the plexiform neurofibroma causing changes in appearance? Has the patient’s mobility changed?
The patient and I have a conversation about their options, including surgery. For some patients, the plexiform neurofibromas are so intertwined with the nerves that complete resection is not possible. Other patients don’t want to undergo surgery. Either way, we discuss how an oral systemic treatment may be appropriate.
Voice-over: When discussing GOMEKLI as an option for patients, I like to go over some of the results from the phase 2b, single-arm ReNeu trial. The data can be complex, so I focus on explaining it in a way that’s clear and relatable. I mention that both adults and children with NF1-PN were included, that they received GOMEKLI for about 2 years, and that they had the option to remain on GOMEKLI for long-term follow-up.
Text onscreen:
114 patients
58 adult patients (18 to 69 years)
56 pediatric patients (2 to 17 years)
Voice-over:
Dr. Weintraub: The primary endpoint was confirmed overall response rate, and I explain that a confirmed overall response meant a person treated with GOMEKLI had 2 or more consecutive MRI scans showing that their plexiform shrank by 20% or more.
Text onscreen:
Primary endpoint
Confirmed overall response (ORR), defined as the proportion of patients with complete response (disappearance of the target PN) or partial response (≥20% reduction) on magnetic resonance imaging (MRI) of the target PN volume from baseline to Cycle 24 (treatment phase) as assessed by blinded independent central review (BICR) on ≥2 consecutive scans within 2 to 6 months.
Voice-over:
Dr. Weintraub: I also note that secondary endpoints included duration of response and change in patient-reported outcomes of tumor pain severity, pain interference, and health-related quality of life.
In the ReNeu clinical trial, 41% of adult patients—or 24 out of 58—saw their plexiform neurofibroma shrink by at least 20%. Of these 24 patients, 62% achieved a deep response, meaning their target plexiform neurofibroma decreased by more than half the size it was at baseline.
Text onscreen:
Graphic of confirmed overall response rates in adults.
Voice-over:
Dr. Weintraub: In the pediatric cohort, 52% of patients—or 29 out of 56—achieved a confirmed overall response. Of these 29 patients, more than half—52%—achieved a deep response.
Text onscreen:
Graphic of confirmed overall response rates in children.
Voice-over:
Dr. Weintraub: I think it’s important to set realistic expectations about how GOMEKLI may help, so I tailor the conversation to the individual characteristics and needs of the patient. I also underscore that not everyone will have the same results.
Dr. Nghiemphu: Yeah, it’s so important to remind patients that clinical trial data in the aggregate is one thing and their personal experience on treatment is another. If patients are expecting to see their plexiform neurofibroma get smaller overnight or in a week, they could get discouraged when that doesn’t happen and want to stop taking their medication. That’s definitely something I want to get ahead of before they start treatment. I highlight that while some patients in the ReNeu trial started to see their plexiform neurofibromas shrink within about 4 months of starting GOMEKLI, for others it took longer. For some it took about 8 months or longer to see a 20% or greater reduction on back-to-back MRI scans. But it’s important that patients stay on track and keep taking their medication as prescribed so that they can get the most out of their treatment.
Dr. Weintraub: That’s a great point. Speaking of staying on track, one question that frequently comes up in my conversations with caregivers is how long their child will have to stay on treatment. I tell them that while every patient is unique, I've had a number of patients who participated in the ReNeu trial and have remained on GOMEKLI treatment ever since.
Text onscreen:
GOMEKLI treatment should be continued until disease progression or unacceptable toxicity.
Text onscreen:
Chapter 2: Reviewing adverse reactions
Text onscreen:
Dr. Phioanh Leia Nghiemphu and Dr. Lauren Weintraub sitting in chairs
Voice-over:
Dr. Nghiemphu: Explaining side effects is a critical part of the initial treatment conversation. I don’t want to overwhelm patients, but I certainly want them to understand what changes, aside from changes in the size of their plexiform neurofibromas, they may experience.
In ReNeu, the most commonly reported adverse reactions in both adults and children were rash, diarrhea, nausea, musculoskeletal pain, and vomiting. Fatigue was also reported in adults.
Image onscreen:
Chart with adult adverse reaction data
Voice-over:
Dr. Nghiemphu: Abdominal pain, headache, paronychia, and left ventricular dysfunction were also reported in children. The most common severe lab abnormalities were increased creatine phosphokinase, or CPK, in both adults and children and decreased neutrophil count in children. Serious side effects associated with GOMEKLI include eye problems, heart problems, skin problems, and embryo-fetal toxicity.
Image onscreen:
Chart with pediatric adverse reaction data
Voice-over:
Dr. Weintraub: I feel it's important to set expectations with patients and caregivers regarding skin-related side effects and counsel them on management strategies. It’s important to address any concerns they may have before starting treatment. Trust me, no teenager wants to be dealing with skin issues on top of living with plexiform neurofibromas.
Dr. Nghiemphu: I definitely have similar conversations with my adult patients about the potential skin-related and gastrointestinal side effects they may experience while on GOMEKLI.
Text onscreen:
Of the patients who had dermatologic adverse reactions in ReNeu, the majority (80%) experienced first onset during Cycle 1 of treatment.
Voice-over:
Dr. Nghiemphu: I make sure they understand that many of these side effects were seen early in the course of treatment and that there are ways to help manage them, including over-the-counter remedies, medications, and even small lifestyle changes.
Text onscreen:
Of the patients who had GI adverse reactions in ReNeu, the majority experienced first onset early (Cycles 1-3).
Text onscreen:
Chart detailing the proportion of patients experiencing GI reactions in the first 2 cycles of GOMEKLI treatment.
Voice-over:
Dr. Nghiemphu: I also discuss how important it is that they are proactive and let me know right away if something is going on. The earlier we can treat the side effect, the better chance we have at resolving it quickly. In addition to managing the side effects, it’s important for patients and caregivers to know that interrupting or reducing their GOMEKLI dose is also an option. And if side effects continue after those adjustments, we have the option to permanently stop GOMEKLI.
Dr. Weintraub: Patients and caregivers should also know that certain assessments, like blood and urine tests, are required before and throughout GOMEKLI treatment. Additionally, because GOMEKLI may cause serious issues with the eyes and heart, eye exams and echocardiograms are also required before and throughout treatment.
Text onscreen:
Icons and check marks next to assessments: blood tests, urinalysis, eye exams, and echocardiograms
Voice-over:
Dr. Weintraub: Generally, these assessments are done more frequently at the start of treatment and become less frequent over time, as things remain stable. I think it’s important for patients and their caregivers to know that these routine assessments help me and their care team monitor how they are doing on treatment. The GOMEKLI Dosing and Adverse Reaction Management Guide outlines some steps we can take as care providers to help manage adverse reactions that occur during GOMEKLI treatment.
Text onscreen:
Animated Dosing and Adverse Reaction Management Guide and QR code.
Text onscreen:
Chapter 3: Dosing, administration, and support
Text onscreen:
Dr. Phioanh Leia Nghiemphu and Dr. Lauren Weintraub sitting in chairs
Voice-over:
Dr. Nghiemphu: How a medication is administered is usually one of the first questions patients have when we’re discussing treatment. GOMEKLI is a twice-daily oral medication that can be taken with or without food. It’s available in 2 capsule sizes and as a dispersible tablet for those who have difficulty swallowing.
Dr. Weintraub: Having the dispersible option is terrific for my patients who can’t or don’t want to take pills. I have a preteen patient who doesn’t like pills and the liquid formulation is just so much easier for him. I also know many caregivers prefer the dispersible tablet because it helps with administering the medication to younger patients.
Dr. Nghiemphu: And of course, some patients have plexiform neurofibromas in the head and neck area that can impact their ability to swallow, so having the option of a dispersible tablet is beneficial to them as well. The dosing schedule for GOMEKLI is 3 weeks on, 1 week off.
Text onscreen:
A chart showing the 4-week dosing cycle
Dr. Nghiemphu: For some patients, having that 1 week off is a nice break because they don’t have to worry about taking treatment. The GOMEKLI Digital Companion is a free and secure resource, available on the Medisafe app, that allows patients to set customized medication reminders, so they never miss a dose.
Dr. Weintraub: We know the coordination of starting a treatment can be a lot for patients and caregivers. SpringWorks CareConnections provides personalized support services to help patients get started and stay on track with GOMEKLI. This includes coverage and access support, financial assistance, personalized education, and emotional support.
Text onscreen:
SpringWorks CareConnections logo and a QR code
Dr. Weintraub: There’s also a full library of resources on GOMEKLI.com that can help set patients up for a positive treatment experience.
Text onscreen:
Additional videos and resources to support your practice and your patients are on GOMEKLI.com/hcp.
Text onscreen:
QR code
Voice-over:
Dr. Nghiemphu: I think we can all agree that when starting a new treatment, it’s important to provide patients with as much information as possible to help set them up for success.
Thank you for listening to our discussion of how we support patients when starting their treatment journey with GOMEKLI.
Voice-over:
Indication
GOMEKLI (mirdametinib) is indicated for the treatment of adult and pediatric patients 2 years of age and older with neurofibromatosis type 1 (NF1) who have symptomatic plexiform neurofibromas (PN) not amenable to complete resection.
Important Safety Information
Warning and Precautions
Ocular Toxicity: GOMEKLI can cause ocular toxicity including retinal vein occlusion (RVO), retinal pigment epithelium detachment (RPED), and blurred vision. In the adult pooled safety population, ocular toxicity occurred in 28% of patients treated with GOMEKLI: 21% were Grade 1, 5% were Grade 2 and 1.3% were Grade 3. RVO occurred in 2.7%, RPED occurred in 1.3%, and blurred vision occurred in 9% of adult patients. In the pediatric pooled safety population, ocular toxicity occurred in 19% of patients: 17% were Grade 1 and 1.7% were Grade 2. Conduct comprehensive ophthalmic assessments prior to initiating GOMEKLI, at regular intervals during treatment, and to evaluate any new or worsening visual changes such as blurred vision. Continue, withhold, reduce the dose, or permanently discontinue GOMEKLI as clinically indicated.
Left Ventricular Dysfunction: GOMEKLI can cause left ventricular dysfunction. GOMEKLI has not been studied in patients with a history of clinically significant cardiac disease or LVEF <55% prior to initiation of treatment. In the ReNeu study, decreased LVEF of 10 to <20% occurred in 16% of adult patients treated with GOMEKLI. Five patients (9%) required dose interruption, one patient (1.7%) required a dose reduction, and one patient required permanent discontinuation of GOMEKLI. The median time to first onset of decreased LVEF in adult patients was 70 days. Decreased LVEF of 10 to <20% occurred in 25%, and decreased LVEF of ≥20% occurred in 1.8% of pediatric patients treated with GOMEKLI. One patient (1.8%) required dose interruption of GOMEKLI. The median time to first onset of decreased LVEF in pediatric patients was 132 days. All patients with decreased LVEF were identified during routine echocardiography, and decreased LVEF resolved in 75% of patients. Before initiating GOMEKLI, assess ejection fraction (EF) by echocardiogram. Monitor EF every 3 months during the first year and then as clinically indicated. Withhold, reduce the dose, or permanently discontinue GOMEKLI based on severity of adverse reaction.
Dermatologic Adverse Reactions: GOMEKLI can cause dermatologic adverse reactions including rash. The most frequent rashes included dermatitis acneiform, rash, eczema, maculo-papular rash and pustular rash. In the pooled adult safety population, rash occurred in 92% of patients treated with GOMEKLI (37% were Grade 2 and 8% were Grade 3) and resulted in permanent discontinuation in 11% of patients. In the pooled pediatric safety population, rash occurred in 72% of patients treated with GOMEKLI (22% were Grade 2 and 3.4% were Grade 3) and resulted in permanent discontinuation in 3.4% of patients. Initiate supportive care at first signs of dermatologic adverse reactions. Withhold, reduce the dose, or permanently discontinue GOMEKLI based on severity of adverse reaction.
Embryo-Fetal Toxicity: GOMEKLI can cause fetal harm when administered to a pregnant woman. Verify the pregnancy status of females of reproductive potential prior to the initiation of GOMEKLI. Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Also advise patients to use effective contraception during treatment with GOMEKLI and for 6 weeks after the last dose (females) or 3 months after the last dose (males).
Adverse Reactions
The most common adverse reactions (>25%) in adult patients were rash (90%), diarrhea (59%), nausea (52%), musculoskeletal pain (41%), vomiting (38%), and fatigue (29%). Serious adverse reactions occurred in 17% of adult patients who received GOMEKLI. The most common Grade 3 or 4 laboratory abnormality (>2%) was increased creatine phosphokinase.
The most common adverse reactions (>25%) in pediatric patients were rash (73%), diarrhea (55%), musculoskeletal pain (41%), abdominal pain (39%), vomiting (39%), headache (34%), paronychia (32%), left ventricular dysfunction (27%), and nausea (27%). Serious adverse reactions occurred in 14% of pediatric patients who received GOMEKLI. The most common Grade 3 or 4 laboratory abnormalities (>2%) were decreased neutrophil count and increased creatine phosphokinase.
Use in Specific Populations
Verify the pregnancy status of patients of reproductive potential prior to initiating GOMEKLI. Due to the potential for adverse reactions in a breastfed child, advise patients not to breastfeed during treatment with GOMEKLI and for 1 week after the last dose.
To report SUSPECTED ADVERSE REACTIONS, contact SpringWorks Therapeutics Inc. at 1-888-400-7989 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see full Prescribing Information, including Patient Information and Instructions for Use.
Text onscreen:
GOMEKLI and SpringWorks logos
Text onscreen:
@2025 SpringWorks Therapeutics, Inc. All rights reserved. GOMEKLI and SpringWorks CareConnections are registered trademarks of SpringWorks Therapeutics, Inc. C_GOM_US_0413 8/25
Helping patients stay on track
Drs. Weintraub and Nghiemphu discuss how to help patients adhere to their GOMEKLI treatment regimen over time.
[Title builds on purple background with GOMEKLI logo in top right corner.]
Text onscreen:
Chapter 1: Getting ready for GOMEKLI
Voice-over:
Dr. Weintraub: Hello. I’m Dr. Lauren Weintraub, a hematologist and oncologist specializing in pediatric cancers and blood disorders.
Text onscreen:
Lauren Weintraub, MD
Dr. Nghiemphu: And I’m Dr. Phioanh Leia Nghiemphu, a neuro-oncologist specializing in neurofibromatosis and brain tumors in adults. Today we’re going to talk about GOMEKLI and how to help patients adhere to their treatment regimen over time.
Text onscreen:
Phioanh Leia Nghiemphu, MD
Dr. Weintraub: GOMEKLI is the first FDA-approved treatment for both adults and children 2 years of age and older with neurofibromatosis type 1 who have symptomatic plexiform neurofibromas that are not amenable to complete resection. Important Safety Information for GOMEKLI will be presented later in this video.
Text onscreen:
GOMEKLI is the first FDA-approved treatment for both adults and children 2 years of age and older with neurofibromatosis type 1 (NF1) who have symptomatic plexiform neurofibromas (PN) that are not amenable to complete resection.
Dr. Nghiemphu: GOMEKLI was evaluated in the ReNeu trial, a large phase 2b, single-arm study that included both adults and children with NF1-PN and lasted for about 2 years and included an optional long-term follow-up phase.
Image onscreen:
Study design graphic
Dr. Nghiemphu: The primary endpoint was confirmed overall response rate, defined as the proportion of patients with a 20% or greater reduction in plexiform neurofibroma volume, as shown on 2 consecutive MRI scans.
Image onscreen: Graphic representation of primary endpoint for both adult and pediatric patients
Dr. Nghiemphu: 41% of adult patients—or 24 out of 58—achieved a confirmed overall response. And 52% of pediatric patients—or 29 out of 56—achieved a confirmed overall response.
Dr. Weintraub: NF1 is a lifelong condition and PNs may require long-term management, so it’s important for patients to understand the short- and long-term treatment experience with GOMEKLI. This presents the opportunity for us to adapt how we support patients, as their needs may change throughout their treatment journey with GOMEKLI.
Text onscreen:
Chapter 2: The early months of treatment
Voiceover:
Dr. Weintraub: When patients start on a long-term treatment like GOMEKLI, I like to sit down with them and their caregivers to set expectations about what those first few months of treatment may look like, including the potential side effects. It’s quite possible that they’ll experience side effects before their plexiform neurofibromas change, and I want them to be prepared for that.
Dr. Nghiemphu: That’s true. While some patients in ReNeu started to experience symptom improvement within a few months on treatment, the median time to onset of confirmed response with GOMEKLI was 7.8 months for adults and 7.9 months for pediatric patients. However, adverse reactions typically occur earlier in the treatment journey.
Image onscreen:
Chart of adult adverse reactions
Dr. Nghiemphu: In ReNeu, the most commonly-reported adverse reactions in both adults and children were rash, diarrhea, nausea, musculoskeletal pain, and vomiting. Fatigue was also reported in adults. Abdominal pain, headache, paronychia, and left ventricular dysfunction were also reported in children.
The most common severe lab abnormalities were increased creatine phosphokinase, or CPK, in both adults and children and decreased neutrophil count in children.
Image onscreen:
Chart of pediatric adverse reactions
Dr. Nghiemphu: Serious side effects associated with GOMEKLI include eye problems, heart problems, skin problems, and embryo-fetal toxicity.
Dr. Weintraub: For me, it’s important to set my patients’ expectations. While they may experience side effects during GOMEKLI treatment, they can—and should—absolutely reach out to me if that happens. I want them to know that this is a safe space for them, and they don’t have to deal with side effects on their own. It’s about making them comfortable enough to discuss what they’re experiencing and tailoring their treatment accordingly.
Dr. Nghiemphu: I also think patients are less discouraged by side effects if they know when they’re likely to occur and that there are ways to manage them. When I talk with patients about skin rash, I tell them: Of those who had skin-related side effects in ReNeu, the majority experienced first onset during Cycle 1—or the first month—of treatment.
Text onscreen:
Of the patients who had dermatologic adverse reactions, the majority (80%) experienced first onset during Cycle 1 of treatment.
Dr. Nghiemphu: I tell them that being proactive with skincare can go a long way. Initiating a prophylactic regimen concurrently with the start of GOMEKLI can help mitigate skin-related adverse reactions.
In addition, I usually suggest daily baths and the use of mild cleansers and skin moisturizers at least twice a day to prevent dryness. I might suggest avoiding certain products that could dry out or irritate the skin.
I also make sure my patients have access to a dermatologist. Since dermatologic adverse reactions are common with GOMEKLI, collaborating with specialists allows us to proactively manage side effects before they become severe.
Dr. Weintraub: I couldn’t agree more. There’s an added challenge with the pediatric population since the types of rashes that develop can depend on the age of the patient. For my young patients, rash tends to be less of an issue, so I don’t necessarily use prophylactic measures. For my preadolescent patients, the acneiform rash can be a major issue, so I prescribe topical therapy for them to have on hand. I also lean on my dermatology colleagues for help because the types of rashes can vary and are sometimes unresponsive to standard therapies.
I have a 12-year-old patient who developed acneiform rash and was very upset about it. I started him on a medication for the rash, and it was working, but I had a backup treatment planned in case it didn’t. I also referred him to a dermatologist in case neither treatment plan worked. I called the dermatologist and explained what NF1-PN is and what my concerns were, and sent him some data so he’d be prepared in case this patient came in.
Being in a more rural area, there’s a bit more education required for other specialists involved in care. They may have never heard of NF1-PN or be familiar with MEK inhibitors and the associated side effects.
Dr. Nghiemphu: I appreciate how proactive you were in setting up that dermatology referral early, so the patient wouldn’t have to wait too long to get an appointment.
Gastrointestinal adverse reactions were also common in the ReNeu trial.
Text onscreen:
Of the patients who had GI adverse reactions in ReNeu, the majority experienced first onset early (Cycles 1-3).
Dr. Nghiemphu: For both adult and pediatric patients, first onset of gastrointestinal reactions were seen during the first 3 cycles of treatment.
Image onscreen:
Chart showing proportion of adult and pediatric patients experiencing GI reactions in first 2 cycles of treatment
Dr. Nghiemphu: To help mitigate side effects like diarrhea, nausea, or vomiting, I advise patients to adjust their diet to avoid fried, fatty, or spicy foods, as well as to increase fluid intake. When they start treatment, I also recommend that they get an over-the-counter antidiarrheal medication, so they have it readily available in case a side effect occurs. As with dermatologic side effects, prophylaxis can be helpful.
Dr. Weintraub: In addition to managing the side effects, it’s important for patients and caregivers to know that interrupting or reducing their GOMEKLI dose is also an option. And if side effects continue after those dose adjustments, we have the option to permanently stop GOMEKLI. For me personally, open and honest conversations with my patients are key. When I know what a patient is experiencing, I can help them navigate through any side effects.
The GOMEKLI Dosing and Adverse Reaction Management Guide is a great resource that includes guidance on managing adverse reactions that occur during treatment. It is available for download at GOMEKLI.com/hcp.
Image onscreen:
Animated Dosing and Adverse Reaction Management Guide and QR code
Text onscreen:
Chapter 3: Long-term needs of patients of taking GOMEKLI
Dr. Nghiemphu: As we discussed earlier, patients may be on a longer treatment journey with GOMEKLI. Sometimes when they’ve been on a medication for a long period, it may be challenging to stay as diligent as at the start of treatment. Let's review some relevant data: As shown in a post hoc exploratory analysis, 62% of adult responders and 52% of pediatric responders achieved a deep response, meaning their target plexiform neurofibroma decreased by more than 50% from baseline.
Image onscreen:
Animation of post hoc exploratory data for adult and pediatric patients
Dr. Nghiemphu: I find it interesting that across both cohorts, patients who achieved a deep response were on GOMEKLI treatment for a longer duration of time than those who did not. That’s why I emphasize to my patients that staying on track with treatment helps increase their chance of benefiting from GOMEKLI.
Text onscreen:
GOMEKLI treatment should be continued until disease progression or unacceptable toxicity
Dr. Weintraub: If you recall, I spoke earlier about a young patient who was experiencing rash. He was so upset he was ready to quit treatment. But while he was focused on the rash, his mother was seeing his plexiform neurofibroma getting softer and smaller. She helped him to recognize the long-term benefits of staying on treatment, and in the meantime, we were able to work through the side effects so he could do just that. I’m always grateful to have parents and caregivers who are so engaged in their child’s care and who help keep them on track with treatment.
In addition to talking about study results, I think it’s helpful for young patients to hear anecdotes like that one. They relate more to stories about what other patients have experienced than to numbers on a page. Although every patient's experience is different, I tell them I have patients who I’ve been treating with GOMEKLI for 5 years, ever since they were first enrolled in the ReNeu trial.
Dr. Nghiemphu: What would you say to a provider who has not prescribed GOMEKLI yet and does not have any of these personal anecdotes to share?
Dr. Weintraub: That’s a great question! I think it goes back to that collaboration we talked about earlier: Reaching out to your colleagues is key. With a complex condition like NF1-PN, you can’t work in a silo. I know we’re all data-driven individuals, but it’s so helpful to hear about the firsthand experience of your peers.
Dr. Nghiemphu: In addition to staying adherent to their treatment regimen, we also want to make sure that patients are coming to their regular appointments. Even if they’re not experiencing symptoms, and their side effects are being managed, regular follow-ups allow me to properly assess their health and make sure things are going well.
Dr. Weintraub: Having a multidisciplinary pediatric clinic definitely makes that easier. We have a support staff that includes NF coordinators, nurses, and case managers all helping to make sure patients follow up with their appointments and get the care they need. We also have a child-life specialist who supports children with the physical challenges that plexiform neurofibromas can bring. Recently, she visited a school to help a patient’s classmates better understand NF1-PN and foster a more supportive, inclusive environment.
Dr. Nghiemphu: Of course, not every clinic has these same resources, and care for adult patients is limited due to the lack of adult NF clinics. For some, even the closest clinic with a multidisciplinary team might still be hard to get to; that's why it's important for clinicians’ offices to meet patients where they are and be flexible with the care model. For patients who live far away, working with a local healthcare provider can help so they don’t have to travel a long distance all the time. If permitted, I sometimes offer virtual visits. If it’s a follow-up to go over lab results, meeting virtually is sufficient and helps ease the travel burden.
Dr. Weintraub: I totally agree. Adapting to a patient’s needs sets the foundation for productive doctor-patient interactions and builds trust. Our patients—no matter how young or old they are—rely on us. And what a great note to end on! Thank you for listening to us discuss how we support patients throughout their treatment journey with GOMEKLI, and be sure to check out the GOMEKLI website for resources for both your practice and patients.
Text onscreen:
Additional videos and resources to support your practice and your patients are on GOMEKLI.com/hcp.
Image onscreen:
QR code
Voiceover:
Indication
GOMEKLI (mirdametinib) is indicated for the treatment of adult and pediatric patients 2 years of age and older with neurofibromatosis type 1 (NF1) who have symptomatic plexiform neurofibromas (PN) not amenable to complete resection.
Important Safety Information
Warning and Precautions
Ocular Toxicity: GOMEKLI can cause ocular toxicity including retinal vein occlusion (RVO), retinal pigment epithelium detachment (RPED), and blurred vision. In the adult pooled safety population, ocular toxicity occurred in 28% of patients treated with GOMEKLI: 21% were Grade 1, 5% were Grade 2 and 1.3% were Grade 3. RVO occurred in 2.7%, RPED occurred in 1.3%, and blurred vision occurred in 9% of adult patients. In the pediatric pooled safety population, ocular toxicity occurred in 19% of patients: 17% were Grade 1 and 1.7% were Grade 2. Conduct comprehensive ophthalmic assessments prior to initiating GOMEKLI, at regular intervals during treatment, and to evaluate any new or worsening visual changes such as blurred vision. Continue, withhold, reduce the dose, or permanently discontinue GOMEKLI as clinically indicated.
Left Ventricular Dysfunction: GOMEKLI can cause left ventricular dysfunction. GOMEKLI has not been studied in patients with a history of clinically significant cardiac disease or LVEF <55% prior to initiation of treatment. In the ReNeu study, decreased LVEF of 10 to <20% occurred in 16% of adult patients treated with GOMEKLI. Five patients (9%) required dose interruption, one patient (1.7%) required a dose reduction, and one patient required permanent discontinuation of GOMEKLI. The median time to first onset of decreased LVEF in adult patients was 70 days. Decreased LVEF of 10 to <20% occurred in 25%, and decreased LVEF of ≥20% occurred in 1.8% of pediatric patients treated with GOMEKLI. One patient (1.8%) required dose interruption of GOMEKLI. The median time to first onset of decreased LVEF in pediatric patients was 132 days. All patients with decreased LVEF were identified during routine echocardiography, and decreased LVEF resolved in 75% of patients. Before initiating GOMEKLI, assess ejection fraction (EF) by echocardiogram. Monitor EF every 3 months during the first year and then as clinically indicated. Withhold, reduce the dose, or permanently discontinue GOMEKLI based on severity of adverse reaction.
Dermatologic Adverse Reactions: GOMEKLI can cause dermatologic adverse reactions including rash. The most frequent rashes included dermatitis acneiform, rash, eczema, maculo-papular rash and pustular rash. In the pooled adult safety population, rash occurred in 92% of patients treated with GOMEKLI (37% were Grade 2 and 8% were Grade 3) and resulted in permanent discontinuation in 11% of patients. In the pooled pediatric safety population, rash occurred in 72% of patients treated with GOMEKLI (22% were Grade 2 and 3.4% were Grade 3) and resulted in permanent discontinuation in 3.4% of patients. Initiate supportive care at first signs of dermatologic adverse reactions. Withhold, reduce the dose, or permanently discontinue GOMEKLI based on severity of adverse reaction.
Embryo-Fetal Toxicity: GOMEKLI can cause fetal harm when administered to a pregnant woman. Verify the pregnancy status of females of reproductive potential prior to the initiation of GOMEKLI. Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Also advise patients to use effective contraception during treatment with GOMEKLI and for 6 weeks after the last dose (females) or 3 months after the last dose (males).
Adverse Reactions
The most common adverse reactions (>25%) in adult patients were rash (90%), diarrhea (59%), nausea (52%), musculoskeletal pain (41%), vomiting (38%), and fatigue (29%). Serious adverse reactions occurred in 17% of adult patients who received GOMEKLI. The most common Grade 3 or 4 laboratory abnormality (>2%) was increased creatine phosphokinase.
The most common adverse reactions (>25%) in pediatric patients were rash (73%), diarrhea (55%), musculoskeletal pain (41%), abdominal pain (39%), vomiting (39%), headache (34%), paronychia (32%), left ventricular dysfunction (27%), and nausea (27%). Serious adverse reactions occurred in 14% of pediatric patients who received GOMEKLI. The most common Grade 3 or 4 laboratory abnormalities (>2%) were decreased neutrophil count and increased creatine phosphokinase.
Use in Specific Populations
Verify the pregnancy status of patients of reproductive potential prior to initiating GOMEKLI. Due to the potential for adverse reactions in a breastfed child, advise patients not to breastfeed during treatment with GOMEKLI and for 1 week after the last dose.
To report SUSPECTED ADVERSE REACTIONS, contact SpringWorks Therapeutics Inc. at 1-888-400-7989 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see full Prescribing Information, including Patient Information and Instructions for Use.
Image onscreen:
GOMEKLI and SpringWorks logos
Text onscreen:
@2025 SpringWorks Therapeutics, Inc. All rights reserved. GOMEKLI is a registered trademark of SpringWorks Therapeutics, Inc. C_GOM_US_0414 8/25
Clinical management of NF1-PN
Drs. Nghiemphu and Moertel discuss the unmet needs and challenges in managing patients with neurofibromatosis type 1 with plexiform neurofibromas (NF1-PN) and factors to consider when determining treatment options.
GOMEKLI logo.
Episode #1: Clinical Management of NF1-PN
The first FDA-approved treatment for both adult and pediatric patients aged ≥2 years with neurofibromatosis type 1 (NF1) who have symptomatic plexiform neurofibromas (PN) not amenable to complete resection.
[Dr Nghiemphu]
My name is Dr Phioanh Leia Nghiemphu, and I’m a neuro-oncologist and the director of the UCLA Neurofibromatosis and Schwannomatosis Clinic.
I’m happy to share today that I also have Dr Moertel, one of the lead investigators of the ReNeu study.
[Dr Moertel]
My name is Dr Christopher Moertel.
I’m a neuro-oncologist and I serve as professor of pediatrics at the University of Minnesota School of Medicine, and I’m the medical director of the Comprehensive Neurofibromatosis Clinic at the University of Minnesota Masonic Children’s Hospital.
[Dr Nghiemphu]
I’d like to have a brief discussion with Dr Moertel about unmet needs and the clinical management of NF1-PN in our practices.
How we treat symptomatic patients with NF1-PN is always evolving. So, in your opinion, Dr Moertel, what factors are the most important when determining management options for a patient with NF1-PN that is symptomatic and has been deemed in need of intervention?
Do these factors differ between pediatric and adult patients? And, if yes, how do they differ?
[Dr Moertel]
Determining the management of patients with NF1-PN is multifactorial, as you know, as it involves considerations of several treatment options to reduce or prevent the morbidities associated with PNs.
When deciding whether or not surgery is appropriate, factors that should be considered include the size and location of the tumor and amenability to resection.
Up to approximately 85% of PNs are not amenable to complete resection.
Additionally, many patients experience postoperative tumor regrowth, pain, and complications of the surgery.
When selecting a systemic treatment, contraindications or preexisting conditions, the age of the patient, and a patient’s ability to swallow pills are considerations.
[Dr Nghiemphu]
What are some of the challenges in managing this disease in your patients?
[Dr Moertel]
Well, as we just mentioned, the rate of PN regrowth after partial, or even complete, resection is one of the many challenges of surgery.
[Dr Moertel, voiceover only]
For systemic treatment, until now, there was no FDA-approved option to treat adult patients with NF1-PN or for individuals who have difficulty swallowing intact capsules. And that’s a lot of people in my practice.
Until now, treatment options for adults were limited to surgery or off-label therapies.
[Dr Moertel]
Having an FDA-approved treatment option for adults is a great step forward.
Dr Nghiemphu, is it important to address the symptomatic plexiform neurofibromas in a timely manner? And, if so, can you explain why it’s important?
[Dr Nghiemphu]
Yes, the clinical manifestation of NF1 can start soon after birth and continue throughout childhood and adulthood.
These manifestations can affect the skeletal system, the skin, and the central nervous system. And NF1-PNs, which can occur at any time throughout life, can be debilitating.
[Dr Nghiemphu, voiceover only]
They are associated with significant morbidities, such as pain, disfigurement, compression of internal organs, and impaired physical function.
The most rapid growth of NF1-PNs usually occurs among those aged 5 years or younger. However, although some patients may experience slow PN regrowth, clinical morbidities often persist and can have a detrimental impact.
[Dr Moertel]
Dr Nghiemphu, of the symptoms experienced with plexiform neurofibromas that you see, what are the most common among your patients?
[Dr Nghiemphu]
For many of my patients, pain can interfere with daily functioning, and the disfigurement can have a substantial effect on their lives.
Reducing the size of PNs may help address these morbidities.
[Dr Moertel, voiceover only]
I would like to review the highlights of the Important Safety Information.
GOMEKLI can cause ocular toxicity, including retinal vein occlusion, retinal pigment epithelium detachment, and blurred vision.
Conduct comprehensive ophthalmic assessments prior to initiating GOMEKLI, at regular intervals during treatment, and to evaluate any new or worsening visual changes.
Follow the dose modification or discontinuation guidance from the Prescribing Information as needed.
GOMEKLI can cause left ventricular dysfunction.
It can occur in patients who are treated with GOMEKLI.
Before initiating GOMEKLI, assess ejection fraction by echocardiogram and monitor ejection fraction every 3 months during the first year and then as clinically indicated. Withhold, reduce the dose of, or discontinue GOMEKLI, depending on the severity of the adverse reaction.
GOMEKLI can cause dermatologic adverse reactions, including rash.
The most frequent rashes include dermatitis acneiform, rash, eczema, maculo-papular rash, and pustular rash.
Initiate supportive care at first signs of dermatologic adverse reactions and follow the dose modification or discontinuation guidance from the Prescribing Information as needed.
GOMEKLI can cause fetal harm when administered to a pregnant woman.
Verify the pregnancy status of females of reproductive potential prior to the initiation of GOMEKLI.
Because of the potential for adverse reactions in breastfed children, women should be advised not to breastfeed during treatment with GOMEKLI and for 1 week after the last dose.
Advise females of reproductive potential to use effective contraception during treatment with GOMEKLI and for 6 weeks after the last dose.
Advise male patients with female partners of reproductive potential to use effective contraception during treatment and for 3 months after the last dose of GOMEKLI.
Clinical overview in adults and case-based insights
Drs. Nghiemphu and Moertel review the ReNeu efficacy and safety results and present 2 case studies of adult patients who were treated with GOMEKLI.
GOMEKLI logo.
Episode #2: Clinical Overview in Adults and Case-Based Insights
[Dr Nghiemphu]
My name is Dr Phioanh Leia Nghiemphu, and I’m a neuro-oncologist and the director of the UCLA Neurofibromatosis and Schwannomatosis Clinic.
I’m happy to share today that I also have Dr Moertel, one of the lead investigators of the ReNeu study.
[Dr Moertel]
My name is Dr Christopher Moertel.
I’m a neuro-oncologist and I serve as professor of pediatrics at the University of Minnesota School of Medicine, and I’m the medical director of the Comprehensive Neurofibromatosis Clinic at the University of Minnesota Masonic Children’s Hospital.
[Dr Nghiemphu]
GOMEKLI is the first FDA-approved treatment for both adult and pediatric patients 2 years of age and older with NF1 who have symptomatic PNs not amenable to complete resection.
At this time, I’d like to pass things over to Dr Moertel, who will give an overview of the ReNeu study for GOMEKLI.
[Dr Moertel]
Thank you!
I’m thrilled to be sharing an overview of the pivotal trial that led to the approval of GOMEKLI.
ReNeu is one of the largest multicenter studies to date of patients with NF1 plexiform neurofibromas.
The efficacy and safety of GOMEKLI were assessed in 114 adult and pediatric patients aged 2 years and older with plexiform neurofibromas causing significant morbidity.
ReNeu was a multicenter, single-arm, phase 2b study with a treatment phase of approximately 22 months, and an optional long-term follow-up treatment phase for those patients who chose to continue treatment with GOMEKLI and a 30-day safety follow-up after treatment discontinuation.
Patients received GOMEKLI based on body surface area at a dosage of 2 mg/m2 orally twice daily on a 3-weeks-on and 1-week-off schedule.
The maximum daily dose of GOMEKLI was 4 mg twice daily.
In both cohorts, the majority of the adult and pediatric patients who completed the treatment phase, with or without a confirmed overall response, chose to remain on GOMEKLI during the long-term follow-up. 84% of adult patients and 85% of pediatric patients made this choice.
The primary endpoint was confirmed overall response rate, which was defined as the proportion of patients with complete response, that is disappearance of the target plexiform neurofibroma, or partial response, a 20% or greater reduction on magnetic resonance imaging of the target PN volume, from baseline to Cycle 24.
That’s the treatment phase, and this was assessed by blinded independent central review on 2 or more consecutive scans within 2 to 6 months.
One of the key features of the ReNeu study was that all responses were assessed by blinded independent central review, and MRIs were reviewed by 2 independent radiologists—concordance in response categorization between reviewers was actually very high.
In this study, the secondary endpoints consisted of safety, tolerability, duration of response, and the change from baseline at prespecified Cycle 13 in patient-reported outcomes, or PROs, of worst tumor pain severity, pain interference, and health-related quality of life.
Dr Nghiemphu, I’ll turn it over to you to present the efficacy data for the adults in the ReNeu study.
[Dr Nghiemphu]
During the treatment phase, 41% of adult patients who were treated with GOMEKLI achieved a confirmed overall response by REiNS criteria.
Based on a post hoc exploratory analysis, among those patients who achieved a confirmed overall response, 62% achieved a deep response, so that’s defined as greater than 50% reduction in the target PN volume from baseline.
This waterfall plot depicts the best percentage change from baseline in tumor volume in adult patients who were treated with GOMEKLI. This was a prespecified exploratory endpoint in the trial.
The teal bars represent adult patients who had a confirmed overall response. The white bars represented nonresponders who had a best overall response of stable disease or progressive disease.
Adults treated with GOMEKLI saw a 41% median reduction in volume of target PNs, and many achieved notable depths of response, with maximum best change being to 90%.
Furthermore, based on a post hoc subgroup analysis, patients who achieved a deep response had a longer median duration of treatment than those who did not achieve a deep response. The median duration of treatment for patients with a deep response was 37 months compared to 26 months for those with just a 20% to 50% reduction in PN volume.
This swimmer plot shows the treatment duration for each patient. Of the 50 evaluable adult patients, 24 had a partial response and 26 had a best response of stable disease.
Note that stable disease and progressive disease were exploratory endpoints, and data should be interpreted with caution, as ReNeu is a single-arm trial.
88% of confirmed overall responders maintained a response for 12 months or more. The median duration of response has not been reached, as the majority of responders are still experiencing a response.
Almost half of the patients, 46%, had an onset of response at the first assessment around Cycle 5, which was approximately 4 months, and the median time to the first response was 7.8 months.
This safety table represents adverse reactions seen in 20% or more of patients.
The most common adverse reactions occurring in greater than 25% of adult patients were rash, diarrhea, nausea, musculoskeletal pain, vomiting, and fatigue.
The majority of adverse reactions were mild to moderate, and there were low rates of severe, Grade 3 or 4, adverse reactions.
For your reference, and to ensure that you and your patients are provided with the support needed, SpringWorks will have a Dosing and Adverse Reaction Management Guide available. This resource includes information on how to manage some of the most common dermatologic and GI side effects, as well as additional information on other adverse reactions.
In the adult cohort, 31% of patients had a dose interruption, and 17% had a dose reduction due to an adverse reaction.
The most common adverse reactions that required dose interruptions were left ventricular dysfunction and COVID-19. Adverse reactions that required dose reductions included rash.
Additionally, 22% of adult patients permanently discontinued GOMEKLI due to rash, diarrhea, nausea, abdominal pain, alopecia, dry skin, left ventricular dysfunction, cough, wheezing, COVID-19, peripheral swelling, retinal vein occlusion, dizziness, and vomiting.
Prior to initiating GOMEKLI, conduct a comprehensive ophthalmic assessment, assess ejection fraction by echocardiogram, and verify the pregnancy status of females of reproductive potential.
During treatment, monitor ejection fraction every 3 months during the first year, and then as clinically indicated. Conduct comprehensive ophthalmic assessment at regular intervals.
Laboratory abnormalities that worsen from baseline and occurred in 15% or more of patients are shown on this slide.
The most common laboratory abnormality was increased CPK. In addition, decreased neutrophil count was also a commonly observed laboratory abnormality in children.
It is recommended to conduct lab tests prior to initiating treatment and at regular intervals during treatment.
So, now we would like to share case studies of patients who were treated with GOMEKLI in the ReNeu clinical trial.
Before we begin, I would like to note that the profile presented is based on an actual patient who was treated with GOMEKLI. Identifiers have been changed. Every patient’s experience is unique and individual results may vary.
The case I will be sharing with you is an 18-year-old female with a painful plexiform neurofibroma in the right breast.
The patient has skin changes and symptoms since birth, but imaging at the age of 12 ultimately led to the diagnosis of NF1.
She is a college student and is active in the NF community.
She presented with multiple PNs, one of which was growing and also was painful.
The tumor first presented when she was around 16 or 17 as a small nodule that grew over the course of a year.
The tumor was not amenable to resection really, because a mastectomy would have been required. The PN diffused through the right breast and involved much of the skin surface.
As you can see from the image here, the PN is evident by the hyperintense white areas on MRI, and there’s a nodule on the inside and thickened white areas over the skin.
Her primary symptoms were hyperesthesia, or extreme sensitivity due to the diffuse PN all over her breast; pain; skin discoloration; and misshapen breasts.
For this patient, daily living was quite a challenge, and different-sized breasts made getting dressed and bra sizing very difficult for her.
Wearing clothing was painful due to skin irritation from fabric contact, and she was concerned about just living with misshapen breasts.
Her parents were very involved with her care and came to appointments with her.
She has been taking over-the-counter pain medications and, because of her age, her care team consisted of a pediatric oncologist who helped her in the transition to adult care.
She was enrolled in the ReNeu clinical trial at 18, as she had a painful PN that was not able to be resected.
As I mentioned before, this would have required mastectomy.
She received the capsule at 4 mg twice a day.
The results from the trial for this patient are shown here. These are MRI images shown on the slides are 2D visuals and do not capture the total volumetric changes observed.
These were images I provided, but additional images may have been utilized during the trial to assess full volumetrics.
The patient achieved a confirmed overall response with a best target volume reduction from baseline of 84%.
Her adverse reactions included mild nausea and vomiting; mild rash, which was managed with doxycycline and clindamycin ointment on the face; and intermittent erythematous rash and itchiness, which were managed with clobetasol lotion.
During Cycle 4, she did have some mild alopecia that emerged. It was treated but did not resolve.
The patient now feels her appearance has improved and is managing her medication and medical diary.
As a result of the tumor volume reduction, wearing a bra and clothing is more comfortable, as irritation from fabric touching her skin has decreased.
The tumor volume reduction has remained durable, and her PN remains stable.
She also reconsented and is now participating in the long-term follow-up of ReNeu.
[Dr Moertel]
This case I’m about to show you is regarding a 69-year-old male with a large plexiform neurofibroma in the head and neck region.
The patient has been a social drinker and smoker since his teenage years, and he, his mother, and his sister have a history of stroke.
He has no family history of NF1.
He was referred by his primary care physician for stabbing pain in the right side of his jaw and hearing loss, or muffled hearing.
Magnetic resonance imaging (MRI) scans confirmed a large walnut-sized plexiform neurofibroma.
The progressing PN was diffused around his neck and penetrated into the neck muscles, so it was not amenable to resection.
It also extended into the muscles of his jaw and occluded his ear canal.
His pain was waking him up and he was unable to function with what he referred to as his stabbing pain.
The patient was taking over-the-counter medications for pain and his care team consisted of a primary care physician, a dermatologist, an ophthalmologist, and myself.
He enrolled in the ReNeu clinical trial and received the capsule formulation of GOMEKLI dosed at 4 mg twice daily.
Results from the trial are shown here.
MRI images shown on the slide, again, are 2D visuals and do not capture the total volumetric changes observed.
These were the images I provided, but additional images may have been utilized during the trial to assess full volumetrics.
The patient achieved a confirmed overall response with a best target tumor volume reduction from baseline of approximately 73%. As a result of this tumor reduction, hearing and symptoms improved.
He did have adverse reactions.
Adverse reactions included a rash on the scalp, for which he was referred to a dermatologist for oral and topical treatment, and at a follow-up visit, his skin was clearer.
He also had mild asymptomatic CPK increase.
The patient opted to reconsent and participated in the long-term follow-up phase of the ReNeu clinical trial, but he discontinued at Cycle 44 after a recurrent stroke unrelated to his treatment.
The patient resumed his art, published a book that he wrote and illustrated, 14 months though, after discontinuing treatment, his symptoms have started to come back.
[Dr Moertel, voiceover only]
I would like to review the highlights of the Important Safety Information.
GOMEKLI can cause ocular toxicity, including retinal vein occlusion, retinal pigment epithelium detachment, and blurred vision.
Conduct comprehensive ophthalmic assessments prior to initiating GOMEKLI, at regular intervals during treatment, and to evaluate any new or worsening visual changes. Follow the dose modification or discontinuation guidance from the Prescribing Information as needed.
GOMEKLI can cause left ventricular dysfunction. It can occur in patients who are treated with GOMEKLI. Before initiating GOMEKLI, assess ejection fraction by echocardiogram and monitor ejection fraction every 3 months during the first year and then as clinically indicated. Withhold, reduce the dose of, or discontinue GOMEKLI, depending on the severity of the adverse reaction.
GOMEKLI can cause dermatologic adverse reactions, including rash.
The most frequent rashes include dermatitis acneiform, rash, eczema, maculo-papular rash, and pustular rash.
Initiate supportive care at first signs of dermatologic adverse reactions and follow the dose modification or discontinuation guidance from the Prescribing Information as needed.
GOMEKLI can cause fetal harm when administered to a pregnant woman.
Verify the pregnancy status of females of reproductive potential prior to the initiation of GOMEKLI.
Because of the potential for adverse reactions in breastfed children, women should be advised not to breastfeed during treatment with GOMEKLI and for 1 week after the last dose.
Advise females of reproductive potential to use effective contraception during treatment with GOMEKLI and for 6 weeks after the last dose.
Advise male patients with female partners of reproductive potential to use effective contraception during treatment and for 3 months after the last dose of GOMEKLI.
Clinical overview in children and case-based insights
Drs. Nghiemphu and Moertel review the ReNeu efficacy and safety results and present a case study of a pediatric patient who was treated with GOMEKLI.
GOMEKLI logo.
Episode #3: Clinical Overview in Children and Case-Based Insights
[Dr Nghiemphu]
My name is Dr Phioanh Leia Nghiemphu, and I’m a neuro-oncologist and the director of the UCLA Neurofibromatosis and Schwannomatosis Clinic.
I’m happy to share today that I also have Dr Moertel, one of the lead investigators of the ReNeu study.
[Dr Moertel]
My name is Dr Christopher Moertel.
I’m a neuro-oncologist and I serve as professor of pediatrics at the University of Minnesota School of Medicine, and I’m the medical director of the Comprehensive Neurofibromatosis Clinic at the University of Minnesota Masonic Children’s Hospital.
[Dr Nghiemphu]
GOMEKLI is the first FDA-approved treatment for both adult and pediatric patients 2 years of age and older with NF1 who have symptomatic PNs not amenable to complete resection.
At this time, I’d like to pass things over to Dr Moertel who will give an overview of the ReNeu study for GOMEKLI.
[Dr Moertel]
Thank you!
I’m thrilled to be sharing an overview of the pivotal trial that led to the approval of GOMEKLI.
ReNeu is one of the largest multicenter studies to date of patients with NF1 plexiform neurofibromas.
The efficacy and safety of GOMEKLI were assessed in 114 adult and pediatric patients aged 2 years and older with plexiform neurofibromas causing significant morbidity.
ReNeu was a multicenter, single-arm, phase 2b study with a treatment phase of approximately 22 months, and an optional long-term follow-up treatment phase for those patients who chose to continue treatment with GOMEKLI and a 30-day safety follow-up after treatment discontinuation.
Patients received GOMEKLI based on a body surface area at a dosage of 2 mg/m2 orally twice daily on a 3-weeks-on and 1-week-off schedule.
The maximum daily dose of GOMEKLI was 4 mg twice daily.
In both cohorts, the majority of the adult and pediatric patients who completed the treatment phase, with or without a confirmed overall response, chose to remain on GOMEKLI during the long-term follow-up. 84% of adult patients and 85% of pediatric patients made this choice.
The primary endpoint was confirmed overall response rate, which was defined as the proportion of patients with complete response, that is disappearance of the target plexiform neurofibroma, or partial response, a 20% or greater reduction on magnetic resonance imaging of the target PN volume, from baseline to Cycle 24.
That’s the treatment phase, and this was assessed by blinded independent central review on 2 or more consecutive scans within 2 to 6 months.
One of the key features of the ReNeu study was that all responses were assessed by blinded independent central review, and MRIs were reviewed by 2 independent radiologists—concordance in response categorization between reviewers was actually very high.
In this study, the secondary endpoints consisted of safety, tolerability, duration of response, and the change from baseline at prespecified Cycle 13 in patient-reported outcomes, or PROs, of worst tumor pain severity, pain interference, and health-related quality of life.
During the treatment phase, 52% of pediatric patients who were treated with GOMEKLI achieved a confirmed overall response by REiNS criteria.
Based on a post hoc exploratory analysis, among those patients who achieved a confirmed overall response, 52% achieved a deep response, defined as greater than 50% reduction in target PN volume from baseline.
This waterfall plot depicts the best percentage change from baseline in tumor volume in pediatric patients who were treated with GOMEKLI.
This was a prespecified exploratory endpoint in the trial.
The gold bars represent pediatric patients who had a confirmed overall response. The white bars represent nonresponders who had a best overall response of stable disease or, in some cases, progressive disease.
Children treated with GOMEKLI saw a 42% median reduction in volume of target PNs, and many achieved notable depths of response, with the maximum best change being 91%.
Furthermore, based on a post hoc analysis of a subgroup, patients who achieved a deep response had a longer median duration of treatment than those who did not achieve a deep response. The median duration of treatment for patients with a deep response was 27 months compared with 25 months for those with a 20% to 50% reduction in plexiform tumor volume.
The swimmer plot shows the treatment duration for each pediatric patient.
Of the 51 evaluable pediatric patients, 29 had a partial response and 22 had a best response of stable disease.
Note that stable disease and progressive disease were exploratory endpoints, and data should be interpreted with caution, as ReNeu is a single-arm trial.
90% of confirmed overall responders maintained a response for 12 months or more.
The median duration of response has not yet been reached, as the majority of responders are still experiencing response.
Almost half of the patients, 45%, had an onset of response at the first on-treatment assessment at Cycle 5, about 4 months, and the median time to first response was 7.9 months.
Now, I’ll pass it back to Dr Nghiemphu, who will review the safety profile of GOMEKLI.
[Dr Nghiemphu]
This safety table represents adverse reactions seen in 20% or more of patients.
The most common adverse reactions occurring in greater than 25% of pediatric patients were rash, diarrhea, musculoskeletal pain, abdominal pain, vomiting, headache, paronychia, left ventricular dysfunction, and nausea.
The majority of adverse reactions were mild to moderate, and there were low rates of severe, Grade 3 or 4, adverse reactions.
For your reference, and to ensure that you and your patients are provided with the support needed, SpringWorks will have a Dosing and Adverse Reaction Management Guide available. This resource includes information on how to manage some of the most common dermatologic and GI side effects, as well as additional information on other adverse reactions.
In the pediatric cohort, 30% of patients had a dose interruption, and 13% had a dose reduction due to an adverse reaction. The most common adverse reaction that required dose interruption was COVID-19. Adverse reactions that required dose reductions included rash and decreased neutrophil count.
Additionally, 9% of pediatric patients permanently discontinued treatment due to urticaria, rash, abdominal pain, constipation, and diarrhea.
Prior to initiating GOMEKLI, conduct a comprehensive ophthalmic assessment, assess ejection fraction by echocardiogram, and verify the pregnancy status of females of reproductive potential.
During treatment, monitor ejection fraction every 3 months during the first year, and then as clinically indicated. Conduct comprehensive ophthalmic assessments at regular intervals.
Laboratory abnormalities that worsened from baseline and occurred in 15% or more of patients are shown on this slide.
The most common laboratory abnormality was increased CPK. In addition, decreased neutrophil count was also a commonly observed laboratory abnormality in children.
It is recommended to conduct lab tests prior to initiating treatment and at regular intervals during treatment.
[Dr Moertel]
This case I would like to share with you is that of a 7-year-old patient with a large abdominal plexiform neurofibroma.
This patient had no family history of NF and was diagnosed with NF1 as an infant.
The patient presented with a grapefruit-sized abdominal plexiform neurofibroma that extended into his left groin.
The patient did not have any prior surgeries and was counseled actually against resection because of the large and invasive size of the tumor, the risk of regrowth, and risk of permanent damage from the surgery.
The PN caused him significant pain that interfered with his daily activities.
He had difficulty walking and was not able to run.
Although he wanted to play sports, such as football, he didn’t because the risk of being hit in the abdomen was too great.
He could not sit for long periods due to discomfort from the location of the tumor, which made school difficult, and he was awakened at night frequently by pain.
The patient was using over-the counter medications and minimizing his activities, unfortunately, to manage his pain. His care team consisted of a primary care physician, a dermatologist, nurse coordinators, and myself.
He enrolled in the ReNeu clinical trial and received the capsule formulation of GOMEKLI dosed at 2 mg twice daily.
Results from the trial are shown here. MRI images are 2-dimensional and do not capture the total volumetric changes observed.
These were the images I provided, but additional images may have been utilized during the trial to assess full volumetrics.
The patient achieved a confirmed overall response with a best target tumor reduction from baseline of approximately 50%.
As a result of the tumor volume reduction, symptoms have improved.
Adverse reactions during therapy included mild diarrhea; vomiting; mild rash, for which skin creams and bleach baths were recommended; and 1 event of asymptomatic Grade 2 left ventricular ejection fraction, which decreased/improved in the long-term follow-up phase.
He also experienced moderate headaches during the off-treatment week from time to time.
The patient enjoys now attending baseball and hockey events and is now prioritizing academic and social activities.
He opted to reconsent and is participating in the long-term follow-up arm of the ReNeu clinical trial.
This patient remains on GOMEKLI to this day.
[Dr Moertel, voiceover only]
I would like to review the highlights of the Important Safety Information.
GOMEKLI can cause ocular toxicity, including retinal vein occlusion, retinal pigment epithelium detachment, and blurred vision.
Conduct comprehensive ophthalmic assessments prior to initiating GOMEKLI, at regular intervals during treatment, and to evaluate any new or worsening visual changes. Follow the dose modification or discontinuation guidance from the Prescribing Information as needed.
GOMEKLI can cause left ventricular dysfunction. It can occur in patients who are treated with GOMEKLI.
Before initiating GOMEKLI, assess ejection fraction by echocardiogram and monitor ejection fraction every 3 months during the first year and then as clinically indicated. Withhold, reduce the dose of, or discontinue GOMEKLI, depending on the severity of the adverse reaction.
GOMEKLI can cause dermatologic adverse reactions, including rash. The most frequent rashes include dermatitis acneiform, rash, eczema, maculo-papular rash, and pustular rash.
Initiate supportive care at first signs of dermatologic adverse reactions and follow the dose modification or discontinuation guidance from the Prescribing Information as needed.
GOMEKLI can cause fetal harm when administered to a pregnant woman.
Verify the pregnancy status of females of reproductive potential prior to the initiation of GOMEKLI.
Because of the potential for adverse reactions in breastfed children, women should be advised not to breastfeed during treatment with GOMEKLI and for 1 week after the last dose.
Advise females of reproductive potential to use effective contraception during treatment with GOMEKLI and for 6 weeks after the last dose.
Advise male patients with female partners of reproductive potential to use effective contraception during treatment and for 3 months after the last dose of GOMEKLI.
Safety profile and adverse reaction management
Drs. Nghiemphu and Moertel review the safety profile of GOMEKLI and discuss adverse reaction management strategies from their own clinical practice and the ReNeu trial.
GOMEKLI logo.
Episode #4 Safety Profile and Adverse Reaction Management
[Dr Nghiemphu]
My name is Dr Phioanh Leia Nghiemphu, and I’m a neuro-oncologist and the director of the UCLA Neurofibromatosis and Schwannomatosis Clinic.
I’m happy to share today that I also have Dr Moertel, one of the lead investigators of the ReNeu study.
[Dr Moertel]
My name is Dr Christopher Moertel.
I’m a neuro-oncologist and I serve as professor of pediatrics at the University of Minnesota School of Medicine, and I’m the medical director of the Comprehensive Neurofibromatosis Clinic at the University of Minnesota Masonic Children’s Hospital.
[Dr Nghiemphu]
GOMEKLI is the first FDA-approved treatment for both adult and pediatric patients 2 years of age and older with NF1 who have symptomatic PNs not amenable to complete resection.
This safety table represents adverse reactions seen in 20% or more of patients.
The most common adverse reactions occurring in greater than 25% of adult patients were rash, diarrhea, nausea, musculoskeletal pain, vomiting, and fatigue.
The most common adverse reactions occurring in greater than 25% of pediatric patients were rash, diarrhea, musculoskeletal pain, abdominal pain, vomiting, headache, paronychia, left ventricular dysfunction, and nausea.
The majority of adverse reactions were mild to moderate, and there were low rates of severe, Grade 3 or 4, adverse reactions. For your reference, and to ensure that you and your patients are provided with the support needed, SpringWorks will have a Dosing and Adverse Reaction Management Guide available. This resource includes information on how to manage some of the most common dermatologic and GI side effects, as well as additional information on other adverse reactions.
In the adult cohort, 31% of patients had a dose interruption, and 17% had a dose reduction due to an adverse reaction.
The most common adverse reactions that required dose interruptions were left ventricular dysfunction and COVID-19. Adverse reactions that required dose reductions included rash.
Additionally, 22% of adult patients permanently discontinued GOMEKLI due to rash, diarrhea, nausea, abdominal pain, alopecia, dry skin, left ventricular dysfunction, cough, wheezing, COVID-19, peripheral swelling, retinal vein occlusion, dizziness, and vomiting.
In the pediatric cohort, 30% of patients had a dose interruption, and 13% had a dose reduction due to an adverse reaction. The most common adverse reaction that required dose interruption was COVID-19. Adverse reactions that required dose reductions included rash and decreased neutrophil count.
Additionally, 9% of pediatric patients permanently discontinued treatment due to urticaria, rash, abdominal pain, constipation, and diarrhea.
Prior to initiating GOMEKLI, conduct a comprehensive ophthalmic assessment, assess ejection fraction by echocardiogram, and verify the pregnancy status of females of reproductive potential.
During treatment, monitor ejection fraction every 3 months during the first year, and then as clinically indicated. Conduct comprehensive ophthalmic assessment at regular intervals.
Laboratory abnormalities that worsened from baseline and occurred in 15% or more of patients are shown on this slide.
In adults and children, the most common laboratory abnormality was increased CPK. In addition, decreased neutrophil count was also a commonly observed laboratory abnormality in children.
It is recommended to conduct lab tests prior to initiating treatment and at regular intervals.
[Dr Moertel]
Now, I’d like to have a discussion with Dr Nghiemphu around management of adverse reactions.
This is important for ensuring that patients can stay on therapy.
Dr Nghiemphu, in the patient cohort in which you were involved in the ReNeu study, how did you manage skin adverse reactions?
[Dr Nghiemphu]
My patients were able to manage through the skin adverse reactions, and this is an important part of MEK inhibitor therapy.
Of the patients who had skin adverse reactions, the majority experienced the first onset pretty early in Cycle 1.
For intolerable Grade 2 or Grade 3 reactions, you should withhold GOMEKLI until Grade 1 or less, and then resume GOMEKLI at a reduced dose.
For Grade 3 or 4 dermatitis acneiform or nonacneiform rash, you should also withhold GOMEKLI until Grade 1 or lower, and then resume GOMEKLI at a reduced dose.
I provided some general guidance, including hygienic skin care practices such as the use of mild cleansers and hypoallergenic moisturizers at least twice a day to prevent dry skin and to avoid agents such as retinoids that could dry out the skin.
In addition, I have seen that proactive management can help a lot with this.
There were some recommendations presented at the Society of Neuro-Oncology Conference last year.
Not every clinician has easy access to a dermatologist, so it’s important to have some management strategies in house.
Dr Moertel, in a patient cohort in which you were involved in the ReNeu study, how did you manage the gastrointestinal adverse reactions?
[Dr Moertel]
For intolerable Grade 2 or Grade 3 reactions, you should withhold GOMEKLI until Grade 1 or less, and then resume GOMEKLI at a reduced dose.
For Grade 4 reactions, consider discontinuing GOMEKLI altogether.
For managing GI adverse reactions, I followed the guidance from the study protocol, which says to consider the good-old BRAT diet, which consists of bananas, rice, applesauce, and toast or just plain pasta.
Also avoid fried, spicy, or fatty foods, and increase fluid intake.
For noninfectious diarrhea, I used loperamide.
[Dr Moertel, voice over only]
I would like to review the highlights of the Important Safety Information.
GOMEKLI can cause ocular toxicity, including retinal vein occlusion, retinal pigment epithelium detachment, and blurred vision.
Conduct comprehensive ophthalmic assessments prior to initiating GOMEKLI, at regular intervals during treatment, and to evaluate any new or worsening visual changes. Follow the dose modification or discontinuation guidance from the Prescribing Information as needed.
GOMEKLI can cause left ventricular dysfunction. It can occur in patients who are treated with GOMEKLI. Before initiating GOMEKLI, assess ejection fraction by echocardiogram and monitor ejection fraction every 3 months during the first year, and then as clinically indicated. Withhold, reduce the dose of, or discontinue GOMEKLI, depending on the severity of the adverse reaction.
GOMEKLI can cause dermatologic adverse reactions, including rash.
The most frequent rashes include dermatitis acneiform, rash, eczema, maculo-papular rash, and pustular rash.
Initiate supportive care at first signs of dermatologic adverse reactions and follow the dose modification or discontinuation guidance from the Prescribing Information as needed.
GOMEKLI can cause fetal harm when administered to a pregnant woman.
Verify the pregnancy status of females of reproductive potential prior to the initiation of GOMEKLI.
Because of the potential for adverse reactions in breastfed children, women should be advised not to breastfeed during treatment with GOMEKLI and for 1 week after the last dose.
Advise females of reproductive potential to use effective contraception during treatment with GOMEKLI and for 6 weeks after the last dose.
Advise male patients with female partners of reproductive potential to use effective contraception during treatment and for 3 months after the last dose of GOMEKLI.
Dosing and administration
GOMEKLI logo.
Episode #5 GOMEKLI Dosing and Administration
[Dr Moertel]
Hi, my name is Dr Christopher Moertel.
I’m a neuro-oncologist and I serve as a professor of pediatrics at the University of Minnesota School of Medicine, and the medical director of the Comprehensive Neurofibromatosis Clinic at the University of Minnesota Masonic Children’s Hospital.
GOMEKLI is the first treatment approved for both adults and children aged 2 years and older with NF1 who have symptomatic plexiform neurofibromas not amenable to complete resection.
Now, let’s discuss the dosing and administration of GOMEKLI.
GOMEKLI has a dosing schedule of 3 weeks on and 1 week off.
Dosing is based on BSA, and should be dosed at 2 mg/m2.
The maximum dosage of GOMEKLI is 4 mg orally twice daily.
You can see here to the left of the slide the various dosing recommendations for different BSA ranges.
Underneath the top-left table and to the right are tables from the Prescribing Information that provide recommendations around dose reductions and dose modifications to address adverse reactions.
Until now, there was no FDA–approved option for children and adults who have difficulty swallowing capsules.
Now, GOMEKLI is the first treatment option available as a dispersible tablet, and it may be taken with or without food.
Instruct patients that tablets can be swallowed whole or dispersed in drinking water and administered orally as a liquid.
This option may be beneficial for patients who are not able to swallow whole tablets.
GOMEKLI can also be taken as capsules, which must be swallowed whole.
Advise patients not to open them, break them, or chew the capsules.
The capsules come in 2-mg and 1-mg strengths, and the dispersible tablets come in a 1-mg strength.
[Dr Moertel, voiceover only]
I would like to review the highlights of the Important Safety Information.
GOMEKLI can cause ocular toxicity, including retinal vein occlusion, retinal pigment epithelium detachment, and blurred vision.
Conduct comprehensive ophthalmic assessments prior to initiating GOMEKLI, at regular intervals during treatment, and to evaluate any new or worsening visual changes. Follow the dose modification or discontinuation guidance from the Prescribing Information as needed.
GOMEKLI can cause left ventricular dysfunction.
It can occur in patients who are treated with GOMEKLI.
Before initiating GOMEKLI, assess ejection fraction by echocardiogram and monitor ejection fraction every 3 months during the first year and then as clinically indicated. Withhold, reduce the dose of, or discontinue GOMEKLI, depending on the severity of the adverse reaction.
GOMEKLI can cause dermatologic adverse reactions, including rash.
The most frequent rashes include dermatitis acneiform, rash, eczema, maculo-papular rash, and pustular rash.
Initiate supportive care at first signs of dermatologic adverse reactions and follow the dose modification or discontinuation guidance from the Prescribing Information as needed.
GOMEKLI can cause fetal harm when administered to a pregnant woman.
Verify the pregnancy status of females of reproductive potential prior to the initiation of GOMEKLI.
Because of the potential for adverse reactions in breastfed children, women should be advised not to breastfeed during treatment with GOMEKLI and for 1 week after the last dose.
Advise females of reproductive potential to use effective contraception during treatment with GOMEKLI and for 6 weeks after the last dose.
Advise male patients with female partners of reproductive potential to use effective contraception during treatment and for 3 months after the last dose of GOMEKLI.

Getting patients started

Helping patients stay on track

Clinical management of NF1-PN
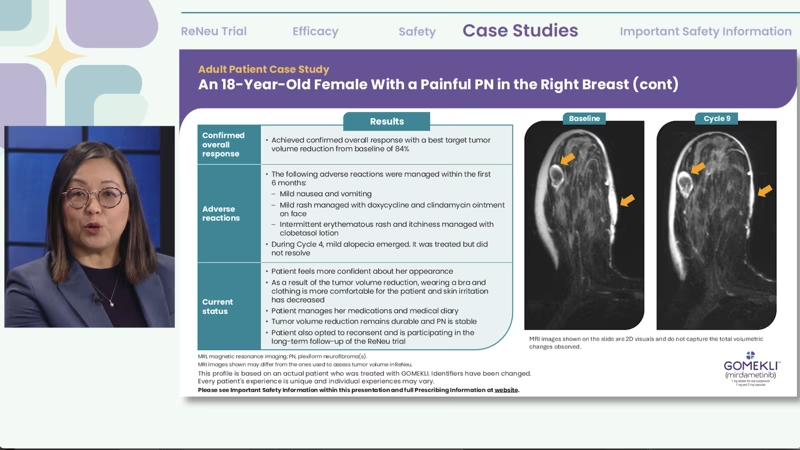
Clinical overview in adults and case-based insights
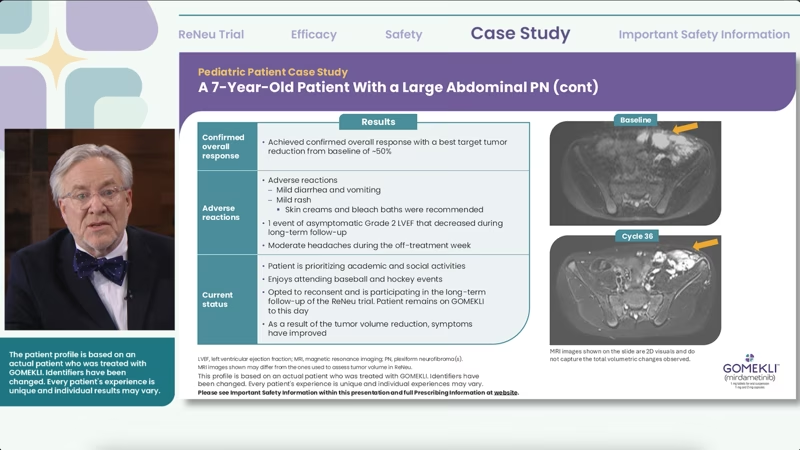
Clinical overview in children and case-based insights

Safety profile and adverse reaction management

Dosing and administration
Austin's GOMEKLI story
Hear Austin, a GOMEKLI Patient Ambassador, talk about neurofibromatosis type 1 with plexiform neurofibromas (NF1-PN) and his experience on GOMEKLI.
- Have eye problems
- Have heart problems
- Are pregnant or plan to become pregnant. GOMEKLI can harm your unborn baby
Females who are able to become pregnant:
- Your healthcare provider should check to see if you are pregnant before you begin treatment with GOMEKLI.
- Use effective birth control (contraception) during treatment with GOMEKLI and for 6 weeks after your last dose.
- Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with GOMEKLI.
- Use effective birth control (contraception) during treatment with GOMEKLI and for 3 months after your last dose.
- Talk to your healthcare provider about the best way to feed your baby during this time.
- Are breastfeeding or plan to breastfeed. It is not known if GOMEKLI passes
into your breastmilk.
- Do not breastfeed during treatment with GOMEKLI and for 1 week after your last dose.
- Talk to your healthcare provider about the best way to feed your baby during this time.
- Take GOMEKLI exactly as your healthcare provider tells you to take it. Your healthcare provider may change your dose, temporarily stop, or permanently stop treatment with GOMEKLI if you develop certain side effects.
- Take GOMEKLI twice a day, about 12 hours apart, for 21 days, followed by 7 days off treatment, to complete a 28-day treatment cycle. Your healthcare provider will decide how many treatment cycles are right for you.
- Take GOMEKLI with or without food.
- GOMEKLI comes in two different dosage forms, GOMEKLI capsules and GOMEKLI tablets for oral suspension. Your healthcare provider will decide the dosage form and dose of GOMEKLI that is right for you.
- If you take GOMEKLI capsules: Swallow each capsule whole with drinking water. If more than 1 capsule is required, swallow 1 capsule at a time. Do not open, break or chew the capsules.
- If you take GOMEKLI tablets for oral suspension, either:
-
- Swallow each tablet for oral suspension whole with drinking water. If more than 1 tablet is required, swallow 1 tablet at a time.
- Disperse the tablets for oral suspension in drinking water to make a liquid (suspension) before you take or give GOMEKLI. See the “Instructions for Use” that come with your medicine for instructions on how to prepare and take GOMEKLI tablets for oral suspension.
-
- If you miss a dose of GOMEKLI, skip the missed dose and take your next dose at your regularly scheduled time.
- If you vomit at any time after taking GOMEKLI, do not take an additional dose. Take your next dose at your regularly scheduled time.
- Eye problems. GOMEKLI may cause eye problems that can lead to blindness.
Your healthcare provider will check your vision before and during treatment
with GOMEKLI. Tell your healthcare provider right away if you get any of the
following signs or symptoms of eye problems:
- Blurred vision
- Loss of vision
- Other changes to your vision
- Heart problems. GOMEKLI may lower the amount of blood pumped by your heart,
which is common in children during treatment with GOMEKLI and can also be
severe. Your healthcare provider will do tests before you start GOMEKLI
treatment, every 3 months during your first year of treatment, and then as
needed to make sure your heart is working properly. Tell your healthcare
provider right away if you get any of the following signs or symptoms of
heart problems:
- Coughing or wheezing
- Shortness of breath
- Swelling of your ankles and feet
- Tiredness
- Increased heart rate
- Skin problems. Skin rashes are common with GOMEKLI in both adults and
children and can also be severe. GOMEKLI can also cause hair loss
(alopecia). Tell your healthcare provider if you develop any of the
following signs or symptoms of skin problems:
- Flat skin rash
- Raised bumps on the skin
- Skin bumps that look like acne
- Skin redness
- Itchy rash
- Peeling skin
- Diarrhea
- Nausea
- Muscle, joint, and bone pain
- Vomiting
- Tiredness
- Diarrhea
- Muscle, joint, and bone pain
- Stomach (abdominal) pain
- Vomiting
- Headache
- Skin redness, swelling, or pain around the fingernails or toenails
- Nausea
The video title and GOMEKLI indication statement appear onscreen with the GOMEKLI logo small in the lower right corner.
Text onscreen:
Meet Austin:
GOMEKLI Guide Patient Ambassador
Text onscreen and voice-over:
What is GOMEKLI?
GOMEKLI (mirdametinib) is a prescription medicine used to treat adults and children 2 years of age and older with neurofibromatosis type 1 (NF1) who have plexiform neurofibromas (PN) that cause symptoms and cannot be completely removed by surgery.
It is not known if GOMEKLI is safe and effective in children under 2 years of age.
Image onscreen:
We follow Austin from behind as he walks into a church, past stained-glass windows, and then down a flight of stairs. We then see him seated at a conference table in a smaller room, looking at a bible, and writing notes in his notebook.
[Austin]
Ministry for me is about showing up for people in their hardest moments. I am there as the shepherd, I’m there to remind them and guide them through those hard times. I’ve been there, I’ve done that, so I know what it means to carry something heavy, invisible, and lifelong. I’ve lived with neurofibromatosis type 1 with plexiform neurofibromas, NF1-PN, for almost my entire life. NF1 is a genetic disorder that causes non-cancerous tumors to grow along the nerves throughout the body.
Image onscreen:
Austin is seated in an interview chair, talking to at an interviewer off-camera. The background shows a living space with plants, a bookshelf, coffee table, artwork, rug, French doors, and a window. A name super graphic appears onscreen.
Text onscreen:
Austin
Living with NF1-PN and taking GOMEKLI® (mirdametinib)
Austin is being compensated by SpringWorks Therapeutics, Inc.
[Austin]
I was two and a half years old when my parents noticed a dark discoloration on my back that looked like a bruise. When it didn't go away, they took me to the doctor. Eventually, scans revealed it was a type of tumor called plexiform neurofibroma and I was diagnosed with NF1-PN. That was the beginning of this journey.
Image onscreen:
Austin is seated at a conference table in a small room. He reads a bible and makes notes in his notebook. There is a mug of tea on the table. In the background are stained-glass windows, a lamp, and a couch. Closeup shots of Austin’s hand holding a pen and writing.
[Austin]
NF1 runs in my family. My dad and grandmother both have it. But no one in the family has tumors like me. By the time I was four, I had to have surgery to remove part of it.
Image onscreen:
Austin is seated in an interview chair, talking to at an interviewer off-camera.
[Austin]
Later that year, I needed another surgery to remove more of that tumor because it was pressing back into my spine. We named my tumor Ralph the Runaway Mouse after one of my favorite children books. It helped us talk about it and normalize it. He’s just a part of me, so to speak.
Image onscreen:
Austin walks along some church pews. He is then shown seated, looking up towards the front of the church, then down at the floor. The video cuts back to the interview shot before showing Austin in the church again.
[Austin]
I was already learning that this condition was not going to be easy to live with. I remember being scared about a few things, but my parents were always honest with me about what was going on. Sometimes doctors would try to pull my parents out of the room, but my parents would tell them that “Austin has to live with this, so he needs to be involved in the conversation.” It helped me be somebody that speaks up and advocates for myself.
Image onscreen:
Austin stands in front of a large table in a room in his house. On the table there are a variety of items associated with camping, such as a lantern, first aid kit, backpack, tarp, tent, etc. He checks over the various items. He then consults a map, planning his hiking route. He packs the items in his backpack, puts on his backpack, grabs some other gear, and then exits his house through the front door. The video occasionally cuts back and forth with the interview shot.
[Austin]
Growing up, NF1-PN affected almost everything in my life. I could not always keep up physically with kids my age, so there was a lot of misunderstanding with coaches and teachers. I remember one time, at a basketball game that I got hit hard right in my tumor. And getting yelled at to get right back up when I was in excruciating pain. People don’t always see what you are carrying. When I got a little older, I started talking to doctors myself. I would tell them where it hurt, and what activities would make it worse. My NF1 specialist, treated me like a person, not just another patient. He understood that I know my own body better than anyone and pushed me to find a treatment that worked for me. We tried several pain regulation treatments to try to manage the symptoms that my plexiforms caused. There were a few years in my early twenties when I was not on treatment. My pain was manageable; I just needed a break from all the appointments. But eventually the pain came back. I was having spasms, and the pain was so intense at times, I could hardly move around. I decided to reengage with care and started seeing a new doctor.
Image onscreen:
Austin carries his backpack and gear outside towards the back of his SUV. The rear lift gate opens automatically, and Austin puts his gear in the trunk. As the trunk closes, the GOMEKLI indication statement appears along the bottom of the screen.
[Austin]
That’s when I heard about a clinical trial for a treatment called mirdametinib, what is now GOMEKLI.
Text onscreen:
GOMEKLI (mirdametinib) is a prescription medicine used to treat adults and children 2 years of age and older with neurofibromatosis type 1 (NF1) who have plexiform neurofibromas that cause symptoms and cannot be completely removed by surgery. It is not known if GOMEKLI is safe and effective in children under 2 years of age.
Image onscreen:
Austin is seated in an interview chair, talking to at an interviewer off-camera. The video then cuts to Austin standing in the forest as he consults his map and compass.
[Austin]
My doctor told me the treatment goal of GOMEKLI was to reduce the size of my plexiform tumors by at least 20%.
Image onscreen:
Austin is seated in an interview chair, talking to at an interviewer off-camera. A disclaimer graphic appears along the bottom of the screen. The video then cuts to a shot following Austin as he hikes along a trail in the forest.
Text onscreen:
Please see Important Safety Information throughout and Full Prescribing Information including Patient Information.
[Austin]
When I heard that, I was just astonished. I was in. I was excited about the idea of my tumor shrinking and hoped that it would relieve some of the symptoms I had because of my tumor.
Image onscreen:
Austin is seated in an interview chair, talking to at an interviewer off-camera. A disclaimer graphic appears along the bottom of the screen.
Text onscreen:
The most common side effects of GOMEKLI in adults include: diarrhea, nausea, muscle, joint, and bone pain, vomiting, tiredness. These are not all of the possible side effects of GOMEKLI. Call your doctor for medical advice about side effects.
[Austin]
Starting GOMEKLI was a commitment. 21 days on, 7 days off, with regular MRIs, and side effects such as rashes and digestive issues, but I worked closely with my doctor to manage these. I learned what worked for me such as staying hydrated and using baby lotion on the rash.
Image onscreen:
We see Austin from the front and side as he hikes along a path in the forest. He uses hiking poles to help with stability.
Text onscreen:
Individual results may vary.
[Austin]
And then, after a year on GOMEKLI, we got the results. A 57% reduction in the size of my tumor.
Image onscreen:
Austin is seated in an interview chair, talking to at an interviewer off-camera. The video then cuts to a wide profile shot of Austin hiking through some long grass, with a forest visible in the background.
[Austin]
I was speechless. It was the first time that something has made a noticeable difference in my NF1-PN. Those results really motivated me to stay on GOMEKLI. This is just my experience, and everyone’s experience may be different.
Image onscreen:
We see Austin in a closeup shot as he drinks water from a reusable water bottle. He then walks into his campsite and sets down a bag on a folding chair. His tent is visible in the background, which is set out on some grass surrounded by trees and bushes. There is a small folding table in front of his chair, as well as a metal firepit stocked with firewood. Austin sets up a portable gas stove and pours water into a pot, which he then puts on the stove to boil. He checks on the water and then sits in his chair, waiting for the water to boil. The video then briefly cuts back to the interview shot.
[Austin]
Now, GOMEKLI is just part of my daily routine. I take it once in the morning and once at night. It doesn’t interfere with my work or the way I want to live my life. If there’s something I could say to someone who is newly diagnosed with NF1-PN, find a doctor who listens to you. Speak up, even if you’re not sure that it matters. And you’re not alone. Reach out to others that are living with NF1-PN. Theres a whole community of us out here that understands.
Image onscreen:
Austin stands at a pulpit in a church and addresses his congregation. Behind Austin, we see lots of dark wood paneling, as well as the wall-mounted silver pipes of an organ. Stained glass windows line the sides of the room. Some shots show the backs of heads and shoulders of people as they listen to Austin preach. The video then fades to white.
[Austin]
Ministry is about being real with people. I show up not in spite of my NF1-PN, but because of it. I know pain, I know frustration. On treatment with GOMEKLI, my tumor is smaller. I have the strength and confidence to focus on the things I love.
Image onscreen:
A disclaimer with the title “Important Safety Information” fades onscreen with the GOMEKLI logo small in the lower right corner. The title and GOMEKLI logo stay in place as the text on screen changes as the presentation of Important Safety Information progresses.
Text onscreen and voice-over:
Important Safety Information
Before taking GOMEKLI, tell your healthcare provider about all of your medical conditions, including if you:
- Have eye problems
- Have heart problems
- Are pregnant or plan to become pregnant. GOMEKLI can harm your unborn baby
Females who are able to become pregnant:- Your healthcare provider should check to see if you are pregnant before you begin treatment with GOMEKLI.
- Use effective birth control (contraception) during treatment with GOMEKLI and for 6 weeks after your last dose.
- Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with GOMEKLI.
- Use effective birth control (contraception) during treatment with GOMEKLI and for 3 months after your last dose.
- Talk to your healthcare provider about the best way to feed your baby during this time.
- Are breastfeeding or plan to breastfeed. It is not known if GOMEKLI passes
into your breastmilk.
- Do not breastfeed during treatment with GOMEKLI and for 1 week after your last dose.
- Talk to your healthcare provider about the best way to feed your baby during this time.
How should I take GOMEKLI?
- Take GOMEKLI exactly as your healthcare provider tells you to take it. Your healthcare provider may change your dose, temporarily stop, or permanently stop treatment with GOMEKLI if you develop certain side effects.
- Take GOMEKLI twice a day, about 12 hours apart, for 21 days, followed by 7 days off treatment, to complete a 28-day treatment cycle. Your healthcare provider will decide how many treatment cycles are right for you.
- Take GOMEKLI with or without food.
- GOMEKLI comes in two different dosage forms, GOMEKLI capsules and GOMEKLI tablets for oral suspension. Your healthcare provider will decide the dosage form and dose of GOMEKLI that is right for you.
- If you take GOMEKLI capsules: Swallow each capsule whole with drinking water. If more than 1 capsule is required, swallow 1 capsule at a time. Do not open, break or chew the capsules.
- If you take GOMEKLI tablets for oral suspension, either:
- Swallow each tablet for oral suspension whole with drinking water. If more than 1 tablet is required, swallow 1 tablet at a time.
- Disperse the tablets for oral suspension in drinking water to make a liquid (suspension) before you take or give GOMEKLI. See the “Instructions for Use” that come with your medicine for instructions on how to prepare and take GOMEKLI tablets for oral suspension.
- If you miss a dose of GOMEKLI, skip the missed dose and take your next dose at your regularly scheduled time.
- If you vomit at any time after taking GOMEKLI, do not take an additional dose. Take your next dose at your regularly scheduled time.
What are the possible side effects of GOMEKLI?
GOMEKLI may cause serious side effects, including:
- Eye problems. GOMEKLI may cause eye problems that can lead to blindness.
Your healthcare provider will check your vision before and during treatment
with GOMEKLI. Tell your healthcare provider right away if you get any of the
following signs or symptoms of eye problems:
- Blurred vision
- Loss of vision
- Other changes to your vision
- Heart problems. GOMEKLI may lower the amount of blood pumped by your heart,
which is common in children during treatment with GOMEKLI and can also be
severe. Your healthcare provider will do tests before you start GOMEKLI
treatment, every 3 months during your first year of treatment, and then as
needed to make sure your heart is working properly. Tell your healthcare
provider right away if you get any of the following signs or symptoms of
heart problems:
- Coughing or wheezing
- Shortness of breath
- Swelling of your ankles and feet
- Tiredness
- Increased heart rate
- Skin problems. Skin rashes are common with GOMEKLI in both adults and
children and can also be severe. GOMEKLI can also cause hair loss
(alopecia). Tell your healthcare provider if you develop any of the
following signs or symptoms of skin problems:
- Flat skin rash
- Raised bumps on the skin
- Skin bumps that look like acne
- Skin redness
- Itchy rash
- Peeling skin
- Diarrhea
- Nausea
- Muscle, joint, and bone pain
- Vomiting
- Tiredness
The most common side effects of GOMEKLI in children include:
- Diarrhea
- Muscle, joint, and bone pain
- Stomach (abdominal) pain
- Vomiting
- Headache
- Skin redness, swelling, or pain around the fingernails or toenails
- Nausea
GOMEKLI may cause fertility problems in females, which may affect your ability to have children. Talk to your healthcare provider if you have concerns about fertility.
These are not all of the possible side effects of GOMEKLI. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Please see full Prescribing Information, including Patient Information and Instructions for Use.
Image onscreen:
The GOMEKLI logo appears large in the center of the screen with disclaimer information in a single line along the bottom.
Text onscreen:
©2025 SpringWorks Therapeutics, Inc. All rights reserved. GOMEKLI is a registered trademark of SpringWorks Therapeutics, Inc. C_GOM_US_0332 07/25

Austin's GOMEKLI story
Indication
GOMEKLI (mirdametinib) is indicated for the treatment of adult and pediatric patients 2 years of age and older with neurofibromatosis type 1 (NF1) who have symptomatic plexiform neurofibromas (PN) not amenable to complete resection.
Important Safety Information
Warnings and Precautions
Ocular Toxicity: GOMEKLI can cause ocular toxicity including retinal vein occlusion (RVO), retinal pigment epithelium detachment (RPED), and blurred vision. In the adult pooled safety population, ocular toxicity occurred in 28% of patients treated with GOMEKLI: 21% were Grade 1, 5% were Grade 2 and 1.3% were Grade 3. RVO occurred in 2.7%, RPED occurred in 1.3%, and blurred vision occurred in 9% of adult patients. In the pediatric pooled safety population, ocular toxicity occurred in 19% of patients: 17% were Grade 1 and 1.7% were Grade 2. Conduct comprehensive ophthalmic assessments prior to initiating GOMEKLI, at regular intervals during treatment, and to evaluate any new or worsening visual changes such as blurred vision. Continue, withhold, reduce the dose, or permanently discontinue GOMEKLI as clinically indicated.
Left Ventricular Dysfunction: GOMEKLI can cause left ventricular dysfunction. GOMEKLI has not been studied in patients with a history of clinically significant cardiac disease or LVEF <55% prior to initiation of treatment. In the ReNeu study, decreased LVEF of 10 to <20% occurred in 16% of adult patients treated with GOMEKLI. Five patients (9%) required dose interruption, one patient (1.7%) required a dose reduction, and one patient required permanent discontinuation of GOMEKLI. The median time to first onset of decreased LVEF in adult patients was 70 days. Decreased LVEF of 10 to <20% occurred in 25%, and decreased LVEF of ≥20% occurred in 1.8% of pediatric patients treated with GOMEKLI. One patient (1.8%) required dose interruption of GOMEKLI. The median time to first onset of decreased LVEF in pediatric patients was 132 days. All patients with decreased LVEF were identified during routine echocardiography, and decreased LVEF resolved in 75% of patients. Before initiating GOMEKLI, assess ejection fraction (EF) by echocardiogram. Monitor EF every 3 months during the first year and then as clinically indicated. Withhold, reduce the dose, or permanently discontinue GOMEKLI based on severity of adverse reaction.
Dermatologic Adverse Reactions: GOMEKLI can cause dermatologic adverse reactions including rash. The most frequent rashes included dermatitis acneiform, rash, eczema, maculo-papular rash and pustular rash. In the pooled adult safety population, rash occurred in 92% of patients treated with GOMEKLI (37% were Grade 2 and 8% were Grade 3) and resulted in permanent discontinuation in 11% of patients. In the pooled pediatric safety population, rash occurred in 72% of patients treated with GOMEKLI (22% were Grade 2 and 3.4% were Grade 3) and resulted in permanent discontinuation in 3.4% of patients. Initiate supportive care at first signs of dermatologic adverse reactions. Withhold, reduce the dose, or permanently discontinue GOMEKLI based on severity of adverse reaction.
Embryo-Fetal Toxicity: GOMEKLI can cause fetal harm when administered to a pregnant woman. Verify the pregnancy status of females of reproductive potential prior to the initiation of GOMEKLI. Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Also advise patients to use effective contraception during treatment with GOMEKLI and for 6 weeks after the last dose (females) or 3 months after the last dose (males).Adverse Reactions
The most common adverse reactions (>25%) in adult patients were rash (90%), diarrhea (59%), nausea (52%), musculoskeletal pain (41%), vomiting (38%), and fatigue (29%). Serious adverse reactions occurred in 17% of adult patients who received GOMEKLI. The most common Grade 3 or 4 laboratory abnormality (>2%) was increased creatine phosphokinase.
The most common adverse reactions (>25%) in pediatric patients were rash (73%), diarrhea (55%), musculoskeletal pain (41%), abdominal pain (39%), vomiting (39%), headache (34%), paronychia (32%), left ventricular dysfunction (27%), and nausea (27%). Serious adverse reactions occurred in 14% of pediatric patients who received GOMEKLI. The most common Grade 3 or 4 laboratory abnormalities (>2%) were decreased neutrophil count and increased creatine phosphokinase.Use in Specific Populations
Indication
References
- GOMEKLI. Prescribing Information. SpringWorks Therapeutics, Inc.
- Rapado F, Simo R, Small M. Neurofibromatosis type 1 of the head and neck: dilemmas in management. J Laryngol Otol. 2001;115(2):151-154.
- Yoo HK, Porteous A, Ng A, et al. Impact of neurofibromatosis type 1 with plexiform neurofibromas on the health-related quality of life and work productivity of adult patients and caregivers in the UK: a cross-sectional survey. BMC Neurology. 2023;23(1):419.