GOMEKLI is a selective and targeted systemic therapy for patients with NF1-PN*
GOMEKLI (mirdametinib) inhibits MEK1/2, key mediators in the MAPK pathway1,2
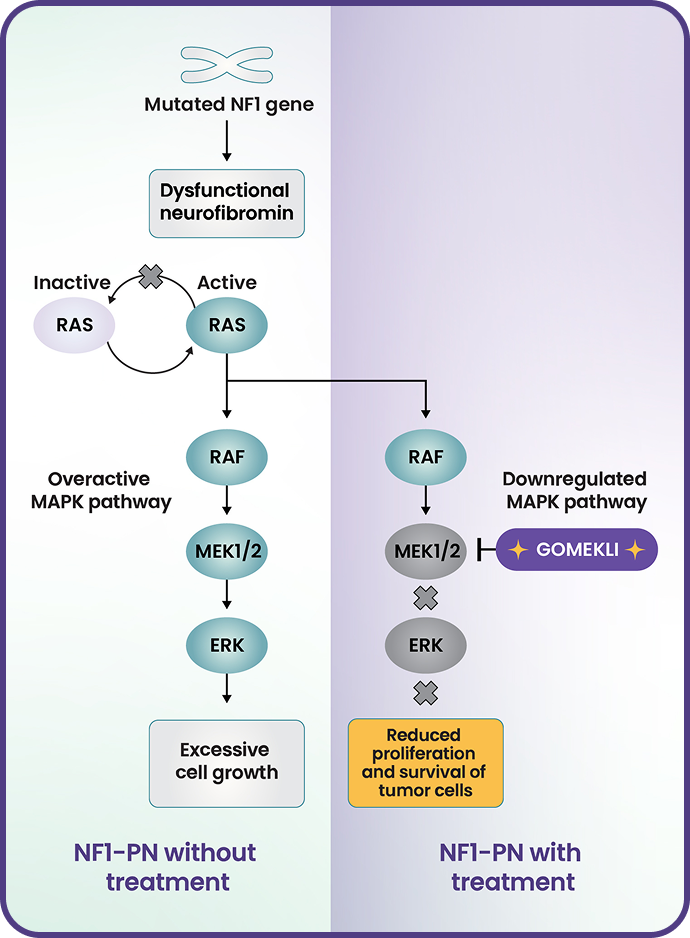
- Neurofibromatosis type 1 (NF1) is caused by loss-of-function variants in the NF1 gene that codes for neurofibromin, a tumor-suppressor protein3,4
- Neurofibromin negatively regulates RAS activity3,4
- In NF1, a lack of functional neurofibromin results in increased RAS signaling and overactivation of the MAPK pathway, which in turn causes uncontrolled cell growth and tumor formation3-5
- In vitro, mirdametinib inhibited kinase activity of MEK1 and MEK2 and downstream phosphorylation of ERK1
- In a mouse model of NF1, mirdametinib was shown to reduce neurofibroma tumor volume and proliferation1
No clinically important drug-drug interactions have been observed with GOMEKLI.1
Learn more.
In in vitro studies:
- GOMEKLI does not inhibit or induce CYP1A2, CYP2B6, CYP2C8, CYP2C9, and CYP2C19, and does not inhibit CYP2D6 or CYP3A4. Some induction potential for CYP3A4 was observed in vitro, but the risk of induction with clinical use at the proposed dose of mirdametinib is low
- GOMEKLI does not inhibit breast cancer resistance protein (BCRP), P-glycoprotein (P-gp), OATP1B1, OATP1B3, OCT2, OAT1, OAT3, MATE1, or MATE2K transporters. Mirdametinib is a substrate of BCRP and P-gp transporters
In a model-informed approach:
- No clinically relevant effects on GOMEKLI exposure were observed with concomitant use of CES1 inhibitors, UGT1A6 inhibitors and inducers, or UGT2B7 inhibitors and inducers evaluated as covariates in a population pharmacokinetic analysis
ERK=extracellular signal-regulated kinase; MAPK=mitogen-activated protein kinase; MEK=mitogen-activated protein kinase kinase; RAF=rapidly accelerated fibrosarcoma; RAS=rat sarcoma viral oncogene homolog.
The efficacy and safety of GOMEKLI were evaluated in both adult and pediatric patients1
Patients had to be ≥2 years of age and have NF1 with a symptomatic, inoperable PN.1,‡
Primary endpoint
Confirmed overall response rate (ORR) defined as the proportion of patients with complete response (disappearance of the target PN) or partial response (≥20% reduction) on magnetic resonance imaging (MRI) of the target PN volume from baseline to Cycle 24 (treatment phase) as assessed by blinded independent central review (BICR) on ≥2 consecutive scans within 2 to 6 months.1
- The first (postbaseline) MRI to assess changes in target PN volume was conducted at Cycle 56
- All responses were assessed by BICR using MRI analysis per Response Evaluation in Neurofibromatosis and Schwannomatosis (REiNS) criteria1
Secondary endpoints1,6
- Duration of response
- Change in patient-reported outcomes, including worst tumor pain severity (NRS-11), pain interference (PII), and HRQoL (PedsQL), from baseline to Cycle 13
- Safety and tolerability
Across adult and pediatric cohorts, the majority of patients who completed the treatment phase (including those with and without a confirmed response) chose to remain on GOMEKLI treatment for long-term follow-up.6
†Patients were required to return for a safety follow-up 30 days after their last dose of study treatment.6
‡Patients were excluded if they had lymphoma, leukemia, or any malignancy (including malignant glioma or MPNST) within the past 5 years; breast cancer within the past 10 years; or evidence of an active optic glioma or other low-grade glioma requiring treatment with chemotherapy or radiation therapy.6
The ReNeu study included adults and children with a broad range of baseline characteristics6
Adult
cohort
(n=58)
cohort
(n=56)
Median age at enrollment (range)
34 years (18-69)
Sex
Male
36%
46%
Female
64%
Target PN progressing at study entry
53%
62%
Location of target PN
48%
50%
Lower extremities
26%
7%
Paraspinal
9%
7%
Chest wall
7%
4%
Mesentery and pelvis
2%
9%
Upper extremities
3%
7%
Abdominal wall
0%
2%
Other
5%
14%
Type of PN-related morbidity§
Pain
90%
70%
Disfigurement or major deformity
52%
50%
Motor dysfunction
40%
27%
Airway dysfunction
5%
12%
Other
17%
21%
Previous PN treatment
Surgery
69%
36%
therapies
19%
14%
Radiotherapy
2%
0%
§Morbidities were clinician determined. Patients may have had more than 1 reported morbidity.
NF1-PN treatment for adults and children
GOMEKLI was proven effective in both adults and children living with NF1-PN.1
Indication
GOMEKLI (mirdametinib) is indicated for the treatment of adult and pediatric patients 2 years of age and older with neurofibromatosis type 1 (NF1) who have symptomatic plexiform neurofibromas (PN) not amenable to complete resection.
Important Safety Information
Warnings and Precautions
Ocular Toxicity: GOMEKLI can cause ocular toxicity including retinal vein occlusion (RVO), retinal pigment epithelium detachment (RPED), and blurred vision. In the adult pooled safety population, ocular toxicity occurred in 28% of patients treated with GOMEKLI: 21% were Grade 1, 5% were Grade 2 and 1.3% were Grade 3. RVO occurred in 2.7%, RPED occurred in 1.3%, and blurred vision occurred in 9% of adult patients. In the pediatric pooled safety population, ocular toxicity occurred in 19% of patients: 17% were Grade 1 and 1.7% were Grade 2. Conduct comprehensive ophthalmic assessments prior to initiating GOMEKLI, at regular intervals during treatment, and to evaluate any new or worsening visual changes such as blurred vision. Continue, withhold, reduce the dose, or permanently discontinue GOMEKLI as clinically indicated.
Left Ventricular Dysfunction: GOMEKLI can cause left ventricular dysfunction. GOMEKLI has not been studied in patients with a history of clinically significant cardiac disease or LVEF <55% prior to initiation of treatment. In the ReNeu study, decreased LVEF of 10 to <20% occurred in 16% of adult patients treated with GOMEKLI. Five patients (9%) required dose interruption, one patient (1.7%) required a dose reduction, and one patient required permanent discontinuation of GOMEKLI. The median time to first onset of decreased LVEF in adult patients was 70 days. Decreased LVEF of 10 to <20% occurred in 25%, and decreased LVEF of ≥20% occurred in 1.8% of pediatric patients treated with GOMEKLI. One patient (1.8%) required dose interruption of GOMEKLI. The median time to first onset of decreased LVEF in pediatric patients was 132 days. All patients with decreased LVEF were identified during routine echocardiography, and decreased LVEF resolved in 75% of patients. Before initiating GOMEKLI, assess ejection fraction (EF) by echocardiogram. Monitor EF every 3 months during the first year and then as clinically indicated. Withhold, reduce the dose, or permanently discontinue GOMEKLI based on severity of adverse reaction.
Dermatologic Adverse Reactions: GOMEKLI can cause dermatologic adverse reactions including rash. The most frequent rashes included dermatitis acneiform, rash, eczema, maculo-papular rash and pustular rash. In the pooled adult safety population, rash occurred in 92% of patients treated with GOMEKLI (37% were Grade 2 and 8% were Grade 3) and resulted in permanent discontinuation in 11% of patients. In the pooled pediatric safety population, rash occurred in 72% of patients treated with GOMEKLI (22% were Grade 2 and 3.4% were Grade 3) and resulted in permanent discontinuation in 3.4% of patients. Initiate supportive care at first signs of dermatologic adverse reactions. Withhold, reduce the dose, or permanently discontinue GOMEKLI based on severity of adverse reaction.
Embryo-Fetal Toxicity: GOMEKLI can cause fetal harm when administered to a pregnant woman. Verify the pregnancy status of females of reproductive potential prior to the initiation of GOMEKLI. Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Also advise patients to use effective contraception during treatment with GOMEKLI and for 6 weeks after the last dose (females) or 3 months after the last dose (males).Adverse Reactions
The most common adverse reactions (>25%) in adult patients were rash (90%), diarrhea (59%), nausea (52%), musculoskeletal pain (41%), vomiting (38%), and fatigue (29%). Serious adverse reactions occurred in 17% of adult patients who received GOMEKLI. The most common Grade 3 or 4 laboratory abnormality (>2%) was increased creatine phosphokinase.
The most common adverse reactions (>25%) in pediatric patients were rash (73%), diarrhea (55%), musculoskeletal pain (41%), abdominal pain (39%), vomiting (39%), headache (34%), paronychia (32%), left ventricular dysfunction (27%), and nausea (27%). Serious adverse reactions occurred in 14% of pediatric patients who received GOMEKLI. The most common Grade 3 or 4 laboratory abnormalities (>2%) were decreased neutrophil count and increased creatine phosphokinase.Use in Specific Populations
Indication
References
- GOMEKLI. Prescribing Information. SpringWorks Therapeutics, Inc.
- Cheng Y, Tian H. Current development status of MEK inhibitors. Molecules. 2017;22(10):1551.
- Gutmann DH, Parada LF, Silva AJ, Ratner N. Neurofibromatosis type 1: modeling CNS dysfunction. J Neurosci. 2012;32(41):14087-14093.
- Gutmann DH, Ferner RE, Listernick RH, Korf BR, Wolters PL, Johnson KJ. Neurofibromatosis type 1. Nat Rev Dis Primers. 2017;3:17004.
- Wang D, Boerner SA, Winkler JD, LoRusso PM. Clinical experience of MEK inhibitors in cancer therapy. Biochim Biophys Acta. 2007;1773(8):1248-1255.
- Moertel CL, Hirbe AC, Shuhaiber HH, et al. ReNeu: a pivotal, phase IIb trial of mirdametinib in adults and children with symptomatic neurofibromatosis type 1-associated plexiform neurofibroma. J Clin Oncol. 2025;43(6):716-729.
- Darrigo LG Jr, Ferraz VEF, Cormedi MCV, et al. Epidemiological profile and clinical characteristics of 491 Brazilian patients with neurofibromatosis type 1. Brain Behav. 2022;12(6):e2599.
- Gross AM, Singh G, Akshintala S, et al. Association of plexiform neurofibroma volume changes and development of clinical morbidities in neurofibromatosis 1. Neuro Oncol. 2018;20(12):1643-1651.

