GOMEKLI (mirdametinib) dosing is designed with patient needs in mind
Actor portrayal.
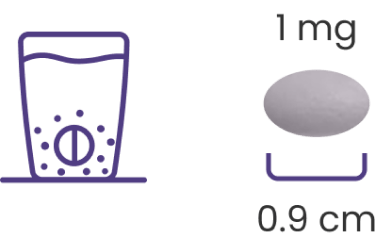
GOMEKLI is available in 2 oral dosing formulations, including a dispersible tablet1
A tablet that can be dispersed in water or swallowed whole
A capsule that comes in 2 sizes

The tablet for oral suspension (dispersible tablet) may help more patients2
Plexiform neurofibromas (PNs) in the head and neck may interfere with the functions of the surrounding anatomical structures, including swallowing. In a study of patients with NF1-PN, 23% (8/35) of adults reported difficulty swallowing.3,4,*
Learn more about how to help patients prepare GOMEKLI for oral suspension
If dosing GOMEKLI as an oral suspension, instruct patients to1:
Gently swirl the water and tablets until the tablets are fully dispersed and an oral suspension is obtained. It takes approximately 2 to 4 minutes to fully disperse the tablets. Once the tablets are dispersed, the oral suspension will appear white and cloudy.
Administer the oral suspension immediately after preparation from a dosing cup or oral syringe.
After administration of the prepared suspension, add approximately 5 mL to 10 mL of drinking water to the dosing cup and gently swirl to resuspend any remaining particles. Administer the suspension to ensure the full dose is taken. Discard the oral suspension if not administered within 30 minutes after preparation.
*An exploratory, cross-sectional, noninterventional study consisting of 2 anonymous surveys directed at adult patients with NF1-PN and caregivers of pediatric patients with NF1-PN.4
Dosing and Administration
Dosing and Administration
Dr. Moertel discusses the dosing and administration of GOMEKLI, the first FDA-approved treatment option for NF1-PN that is available as a dispersible tablet for patients who have difficulty swallowing.1,3,4
GOMEKLI logo.
Episode #5 GOMEKLI Dosing and Administration
[Dr Moertel]
Hi, my name is Dr Christopher Moertel.
I'm a neuro-oncologist and I serve as a professor of pediatrics at the University of Minnesota School of Medicine, and the medical director of the Comprehensive Neurofibromatosis Clinic at the University of Minnesota Masonic Children's Hospital.
GOMEKLI is the first treatment approved for both adults and children aged 2 years and older with NF1 who have symptomatic plexiform neurofibromas not amenable to complete resection.
Now, let's discuss the dosing and administration of GOMEKLI.
GOMEKLI has a dosing schedule of 3 weeks on and 1 week off.
Dosing is based on BSA, and should be dosed at 2 mg/m2.
The maximum dosage of GOMEKLI is 4 mg orally twice daily.
You can see here to the left of the slide the various dosing recommendations for different BSA ranges.
Underneath the top-left table and to the right are tables from the Prescribing Information that provide recommendations around dose reductions and dose modifications to address adverse reactions.
Until now, there was no FDA–approved option for children and adults who have difficulty swallowing capsules.
Now, GOMEKLI is the first treatment option available as a dispersible tablet, and it may be taken with or without food.
Instruct patients that tablets can be swallowed whole or dispersed in drinking water and administered orally as a liquid.
This option may be beneficial for patients who are not able to swallow whole tablets.
GOMEKLI can also be taken as capsules, which must be swallowed whole.
Advise patients not to open them, break them, or chew the capsules.
The capsules come in 2-mg and 1-mg strengths, and the dispersible tablets come in a 1-mg strength.
[Dr Moertel, voiceover only]
I would like to review the highlights of the Important Safety Information.
GOMEKLI can cause ocular toxicity, including retinal vein occlusion, retinal pigment epithelium detachment, and blurred vision.
Conduct comprehensive ophthalmic assessments prior to initiating GOMEKLI, at regular intervals during treatment, and to evaluate any new or worsening visual changes. Follow the dose modification or discontinuation guidance from the Prescribing Information as needed.
GOMEKLI can cause left ventricular dysfunction.
It can occur in patients who are treated with GOMEKLI.
Before initiating GOMEKLI, assess ejection fraction by echocardiogram and monitor ejection fraction every 3 months during the first year and then as clinically indicated. Withhold, reduce the dose of, or discontinue GOMEKLI, depending on the severity of the adverse reaction.
GOMEKLI can cause dermatologic adverse reactions, including rash.
The most frequent rashes include dermatitis acneiform, rash, eczema, maculo-papular rash, and pustular rash.
Initiate supportive care at first signs of dermatologic adverse reactions and follow the dose modification or discontinuation guidance from the Prescribing Information as needed.
GOMEKLI can cause fetal harm when administered to a pregnant woman.
Verify the pregnancy status of females of reproductive potential prior to the initiation of GOMEKLI.
Because of the potential for adverse reactions in breastfed children, women should be advised not to breastfeed during treatment with GOMEKLI and for 1 week after the last dose.
Advise females of reproductive potential to use effective contraception during treatment with GOMEKLI and for 6 weeks after the last dose.
Advise male patients with female partners of reproductive potential to use effective contraception during treatment and for 3 months after the last dose of GOMEKLI.
Dr. Moertel discusses the dosing and administration of GOMEKLI, the first FDA-approved treatment option for NF1-PN that is available as a dispersible tablet for patients who have difficulty swallowing.1,3,4
GOMEKLI is taken on a 3-weeks-on/1-week-off dosing schedule1
- The recommended dose is 2 mg/m2 twice daily, with a maximum dose of 4 mg twice daily
- GOMEKLI can be taken with or without food
Determining a patient’s dose based on body surface area (BSA)1
Recommended dosage for GOMEKLI
Recommended dosage for capsules or tablets for oral suspension
0.40 to 0.69
1 mg twice daily
0.70 to 1.04
2 mg twice daily
1.05 to 1.49
3 mg twice daily
≥1.50
4 mg twice daily
It is important to monitor your patients and reassess dosage based on any BSA changes.
Continue treatment with GOMEKLI until disease progression or unacceptable toxicity.
View recommended dose reductions/modifications for GOMEKLI
Recommended dose reductions for adverse reactions1
BSA (m2)
Reduced dose‡
0.40 to 0.69
2 mg
1 mg
2 mg
2 mg
3 mg
3 mg
Recommended dose modifications for adverse reactions1
Ocular toxicity
Grade ≤2
- Continue GOMEKLI at current dose level
- Consider ophthalmologic examinations every 2 to 4 weeks until resolution to ≤Grade 1 or baseline
Grade ≥3
- Withhold GOMEKLI until ≤Grade 1 or baseline
- If recovery occurs ≤14 days, resume GOMEKLI at the next lower dose
- If recovery occurs in >14 days, consider permanent discontinuation of GOMEKLI
Symptomatic retinal pigment epithelium detachment (RPED)
- Withhold GOMEKLI until ≤Grade 1 or baseline
- Resume GOMEKLI at the same dose
Retinal vein occlusion (RVO)
- Permanently discontinue GOMEKLI
Asymptomatic, absolute decrease in LVEF of 10% or greater from baseline and is less than the lower limit of normal
- Withhold GOMEKLI until ≤Grade 1
- Resume GOMEKLI at reduced dose
- Permanently discontinue GOMEKLI
Intolerable Grade 2 or Grade 3
- Withhold GOMEKLI until ≤Grade 1
- Resume GOMEKLI at reduced dose
Grade 3 or 4 dermatitis acneiform or nonacneiform rash
- Withhold GOMEKLI until ≤Grade 1
- Resume GOMEKLI at reduced dose
Other adverse reactions
Intolerable Grade 2 or Grade 3
- Withhold GOMEKLI until ≤Grade 1
- Resume GOMEKLI at reduced dose
Grade 4
- Consider permanent discontinuation of GOMEKLI
‡Permanently discontinue GOMEKLI in patients unable to tolerate GOMEKLI after one dose reduction.
§Per National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0 (NCI CTCAE v. 5.0).
†The recommended dosage for patients with a BSA less than 0.40 m2 has not been established.1
Routine tests before and during treatment
Pregnancy test
Lab work
Blood tests and urinalysis should be initiated prior to treatment, and at regular intervals during treatment, to assess for abnormalities (ie, changes in serum CPK).2
Ophthalmic assessment
Dosing modifications
The GOMEKLI Dosing and Adverse Reaction Management Guide includes an overview of GOMEKLI dosing and administration as well as guidance on managing adverse reactions that may occur during treatment.
Personalized support
Indication
GOMEKLI (mirdametinib) is indicated for the treatment of adult and pediatric patients 2 years of age and older with neurofibromatosis type 1 (NF1) who have symptomatic plexiform neurofibromas (PN) not amenable to complete resection.
Important Safety Information
Warnings and Precautions
Ocular Toxicity: GOMEKLI can cause ocular toxicity including retinal vein occlusion (RVO), retinal pigment epithelium detachment (RPED), and blurred vision. In the adult pooled safety population, ocular toxicity occurred in 28% of patients treated with GOMEKLI: 21% were Grade 1, 5% were Grade 2 and 1.3% were Grade 3. RVO occurred in 2.7%, RPED occurred in 1.3%, and blurred vision occurred in 9% of adult patients. In the pediatric pooled safety population, ocular toxicity occurred in 19% of patients: 17% were Grade 1 and 1.7% were Grade 2. Conduct comprehensive ophthalmic assessments prior to initiating GOMEKLI, at regular intervals during treatment, and to evaluate any new or worsening visual changes such as blurred vision. Continue, withhold, reduce the dose, or permanently discontinue GOMEKLI as clinically indicated.
Left Ventricular Dysfunction: GOMEKLI can cause left ventricular dysfunction. GOMEKLI has not been studied in patients with a history of clinically significant cardiac disease or LVEF <55% prior to initiation of treatment. In the ReNeu study, decreased LVEF of 10 to <20% occurred in 16% of adult patients treated with GOMEKLI. Five patients (9%) required dose interruption, one patient (1.7%) required a dose reduction, and one patient required permanent discontinuation of GOMEKLI. The median time to first onset of decreased LVEF in adult patients was 70 days. Decreased LVEF of 10 to <20% occurred in 25%, and decreased LVEF of ≥20% occurred in 1.8% of pediatric patients treated with GOMEKLI. One patient (1.8%) required dose interruption of GOMEKLI. The median time to first onset of decreased LVEF in pediatric patients was 132 days. All patients with decreased LVEF were identified during routine echocardiography, and decreased LVEF resolved in 75% of patients. Before initiating GOMEKLI, assess ejection fraction (EF) by echocardiogram. Monitor EF every 3 months during the first year and then as clinically indicated. Withhold, reduce the dose, or permanently discontinue GOMEKLI based on severity of adverse reaction.
Dermatologic Adverse Reactions: GOMEKLI can cause dermatologic adverse reactions including rash. The most frequent rashes included dermatitis acneiform, rash, eczema, maculo-papular rash and pustular rash. In the pooled adult safety population, rash occurred in 92% of patients treated with GOMEKLI (37% were Grade 2 and 8% were Grade 3) and resulted in permanent discontinuation in 11% of patients. In the pooled pediatric safety population, rash occurred in 72% of patients treated with GOMEKLI (22% were Grade 2 and 3.4% were Grade 3) and resulted in permanent discontinuation in 3.4% of patients. Initiate supportive care at first signs of dermatologic adverse reactions. Withhold, reduce the dose, or permanently discontinue GOMEKLI based on severity of adverse reaction.
Embryo-Fetal Toxicity: GOMEKLI can cause fetal harm when administered to a pregnant woman. Verify the pregnancy status of females of reproductive potential prior to the initiation of GOMEKLI. Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Also advise patients to use effective contraception during treatment with GOMEKLI and for 6 weeks after the last dose (females) or 3 months after the last dose (males).Adverse Reactions
The most common adverse reactions (>25%) in adult patients were rash (90%), diarrhea (59%), nausea (52%), musculoskeletal pain (41%), vomiting (38%), and fatigue (29%). Serious adverse reactions occurred in 17% of adult patients who received GOMEKLI. The most common Grade 3 or 4 laboratory abnormality (>2%) was increased creatine phosphokinase.
The most common adverse reactions (>25%) in pediatric patients were rash (73%), diarrhea (55%), musculoskeletal pain (41%), abdominal pain (39%), vomiting (39%), headache (34%), paronychia (32%), left ventricular dysfunction (27%), and nausea (27%). Serious adverse reactions occurred in 14% of pediatric patients who received GOMEKLI. The most common Grade 3 or 4 laboratory abnormalities (>2%) were decreased neutrophil count and increased creatine phosphokinase.Use in Specific Populations
Indication
References
- GOMEKLI. Prescribing Information. SpringWorks Therapeutics, Inc.
- Moertel CL, Hirbe AC, Shuhaiber HH, et al. ReNeu: a pivotal, phase IIb trial of mirdametinib in adults and children with symptomatic neurofibromatosis type 1-associated plexiform neurofibroma. J Clin Oncol. 2025;43(6):716-729.
- Rapado F, Simo R, Small M. Neurofibromatosis type 1 of the head and neck: dilemmas in management. J Laryngol Otol. 2001;115(2):151-154.
- Yoo HK, Porteous A, Ng A, et al. Impact of neurofibromatosis type 1 with plexiform neurofibromas on the health-related quality of life and work productivity of adult patients and caregivers in the UK: a cross-sectional survey. BMC Neurology. 2023;23(1):419.